2025
|
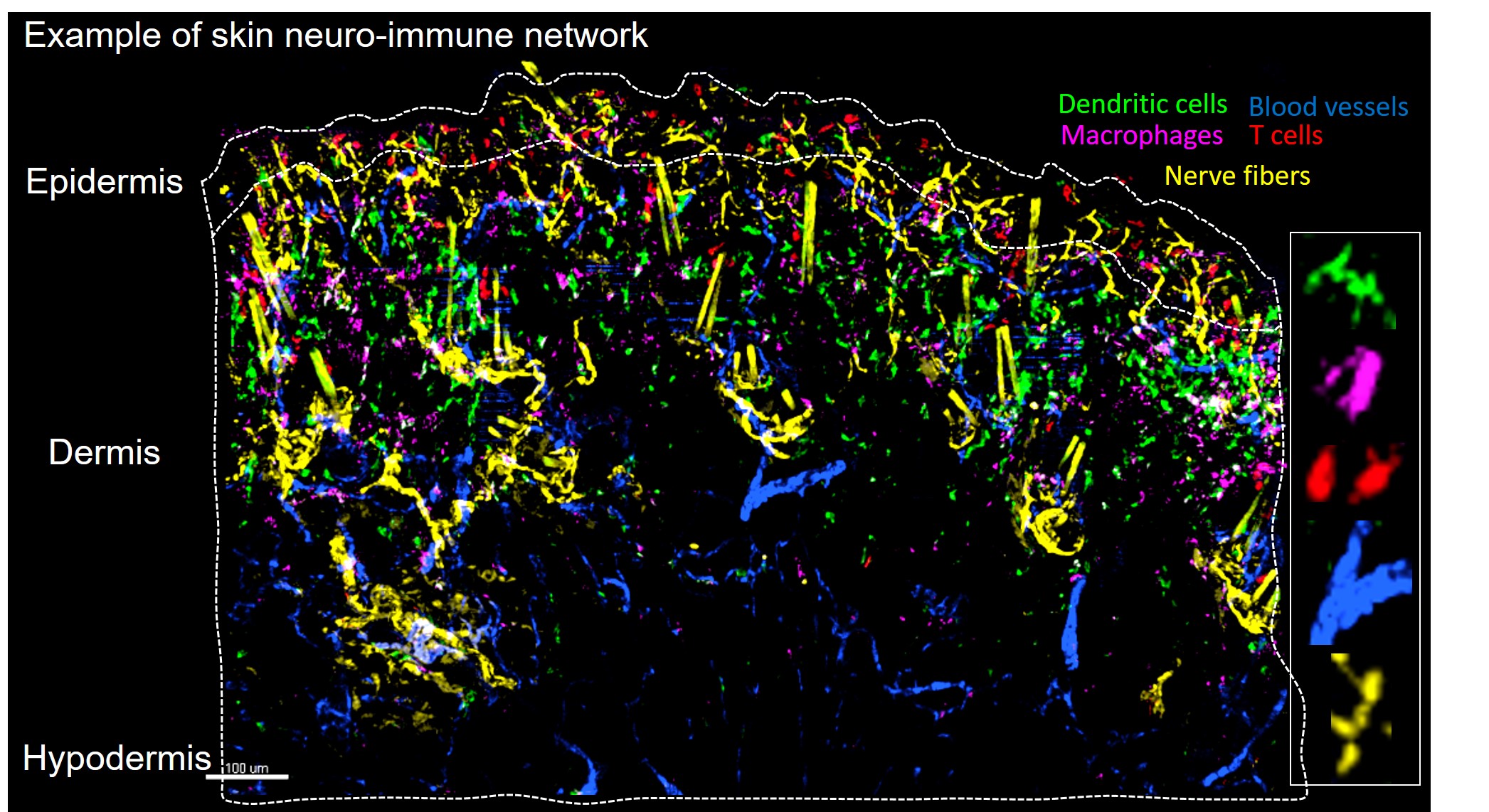
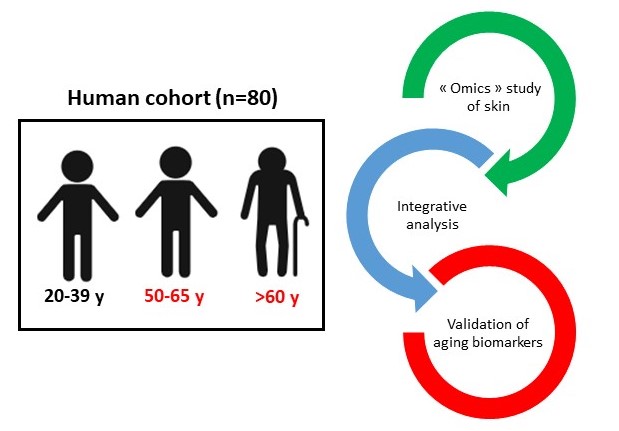
Abdullah NS Serhan N, Gheziel N Maternal stress triggers early-life eczema through fetal mast cell programming Article de journal Dans: Nature, 2025. @article{serhan2025,
title = {Maternal stress triggers early-life eczema through fetal mast cell programming},
author = {Serhan N, Abdullah NS, Gheziel N, Loste A, Ekren R, Labit E, Gonzalez AA, Oliva G, Tarot P, Petitfils C, Payros G, D'Avino P, Voisin A, Tinsley HFG, Gentek R, Brosseau C, Bodinier M, Reber L, Val P, Akdis CA, Mitamura Y, Andiappan AK, Chan JKY, Ginhoux F, François A, Cénac N, Basso L, Gaudenzio N},
doi = {https://doi.org/10.1038/s41586-025-09419-8},
year = {2025},
date = {2025-08-27},
journal = {Nature},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2024
|
Starkl, Philipp; Jonsson, Gustav; Artner, Tyler; Turnes, Bruna Lenfers; Gail, Laura-Marie; Oliveira, Tiago; Jain, Aakanksha; Serhan, Nadine; Stejskal, Karel; Lakovits, Karin; Hladik, Anastasiya; An, Meilin; Channon, Keith M.; Kim, Hail; Köcher, Thomas; Weninger, Wolfgang; Stary, Georg; Knapp, Sylvia; Klang, Victoria; Gaudenzio, Nicolas; Woolf, Clifford J.; Tikoo, Shweta; Jain, Rohit; Penninger, Josef M.; Cronin, Shane J. F. Mast cell–derived BH4 and serotonin are critical mediators of postoperative pain Article de journal Dans: Science Immunology, 2024. @article{Starkl2024,
title = {Mast cell–derived BH4 and serotonin are critical mediators of postoperative pain},
author = {Philipp Starkl and Gustav Jonsson and Tyler Artner and Bruna Lenfers Turnes and Laura-Marie Gail and Tiago Oliveira and Aakanksha Jain and Nadine Serhan and Karel Stejskal and Karin Lakovits and Anastasiya Hladik and Meilin An and Keith M. Channon and Hail Kim and Thomas Köcher and Wolfgang Weninger and Georg Stary and Sylvia Knapp and Victoria Klang and Nicolas Gaudenzio and Clifford J. Woolf and Shweta Tikoo and Rohit Jain and Josef M. Penninger and Shane J.F. Cronin},
url = {https://www-science-org.proxy.insermbiblio.inist.fr/doi/10.1126/sciimmunol.adh0545},
doi = {10.1126/sciimmunol.adh0545},
year = {2024},
date = {2024-08-23},
urldate = {2024-08-23},
journal = {Science Immunology},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Scholaert M; Peries M; Braun E; Martin J; Serhan N; Loste A;, Bruner A; Basso L; Chaput B; Merle E; Descargues P; Pagès E; Gaudenzio N Multimodal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine Article de journal Dans: Allergy, 2024. @article{Scholaert2024,
title = {Multimodal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine},
author = {Scholaert M; Peries M; Braun E; Martin J; Serhan N; Loste A;, Bruner A; Basso L; Chaput B; Merle E; Descargues P; Pagès E; Gaudenzio N},
doi = {https://doi.org/10.1111/all.16273},
year = {2024},
date = {2024-08-19},
urldate = {2024-08-19},
journal = {Allergy},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Voisin, Tiphaine; Labit, Elodie; Gaudenzio, Nicolas; Basso, Lilian Anaplastic lymphoma kinase as a new therapeutic target in inflammatory itch Article de journal Dans: Allergy, 2024. @article{Voisin2024,
title = {Anaplastic lymphoma kinase as a new therapeutic target in inflammatory itch},
author = {Tiphaine Voisin and Elodie Labit and Nicolas Gaudenzio and Lilian Basso},
url = {https://onlinelibrary-wiley-com.proxy.insermbiblio.inist.fr/doi/full/10.1111/all.16260},
doi = { https://doi-org.proxy.insermbiblio.inist.fr/10.1111/all.16260},
year = {2024},
date = {2024-07-26},
journal = {Allergy},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Wollam, Joshua; Solomon, Michelle; Villescaz, Christiane; Lanier, Marion; Evans, Samantha; Bacon, Corinne; Freeman, David; Vasquez, Alexis; Vest, Alan; Napora, Jim; Charlot, Brittney; Cavarlez, Christine; Kim, Andrew; Dvorak, Lisa; Selfridge, Brandon; Huang, Liming; Nevarez, Andres; Dedman, Harry; Brooks, Jennifer; Frischbutter, Stefan; Metz, Martin; Serhan, Nadine; Gaudenzio, Nicolas; Timony, Gregg; Martinborough, Esther; Boehm, Marcus F.; Viswanath, Veena Inhibition of Mast Cell Degranulation by Novel Small Molecule MRGPRX2 Antagonists Article de journal Dans: Journal of Allergy and Clinical Immunology, 2024. @article{Wollam2024,
title = {Inhibition of Mast Cell Degranulation by Novel Small Molecule MRGPRX2 Antagonists},
author = {Joshua Wollam and Michelle Solomon and Christiane Villescaz and Marion Lanier and Samantha Evans and Corinne Bacon and David Freeman and Alexis Vasquez and Alan Vest and Jim Napora and Brittney Charlot and Christine Cavarlez and Andrew Kim and Lisa Dvorak and Brandon Selfridge and Liming Huang and Andres Nevarez and Harry Dedman and Jennifer Brooks and Stefan Frischbutter and Martin Metz and Nadine
Serhan and Nicolas Gaudenzio and Gregg Timony and Esther Martinborough and Marcus F. Boehm and Veena Viswanath},
url = {https://www.jacionline.org/article/S0091-6749(24)00675-4/fulltext},
year = {2024},
date = {2024-07-04},
urldate = {2024-07-04},
booktitle = {Journal of Allergy and Clinical Immunology},
journal = {Journal of Allergy and Clinical Immunology},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2023
|
Scholaert, Manon; Peries, Mathias; Braun, Emilie; Martin, Jeremy; Serhan, Nadine; Loste, Alexia; Bruner, Audrey; Basso, Lilian; Chaput, Benoit; Merle, Eric; Descargues, Pascal; Pages, Emeline; Gaudenzio, Nicolas Pre-print | Multi-modal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine Article de journal Dans: bioRxiv, 2023. @article{Scholaert2023,
title = {Pre-print | Multi-modal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine},
author = {Manon Scholaert and Mathias Peries and Emilie Braun and Jeremy Martin and Nadine Serhan and Alexia Loste and Audrey Bruner and Lilian Basso and Benoit Chaput and Eric Merle and Pascal Descargues and Emeline Pages and Nicolas Gaudenzio},
url = {https://www.biorxiv.org/content/10.1101/2023.09.22.558940v1},
year = {2023},
date = {2023-09-27},
journal = {bioRxiv},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Tauber#, Marie; Basso#, Lilian; Martin#, Jeremy; Bostan, Luciana; Pinto, Marlene Magalhaes; Thierry, Guilhem R; Houmadi, Raïssa; Serhan, Nadine; Loste, Alexia; Blériot, Camille; Kamphuis, Jasper B J; Grujic, Mirjana; Kjellén, Lena; Pejler, Gunnar; Paul, Carle; Dong, Xinzhong; Galli, Stephen J; Reber, Laurent L; Ginhoux, Florent; Bajenoff, Marc; Gentek, Rebecca; Gaudenzio, Nicolas Landscape of mast cell populations across organs in mice and humans Article de journal Dans: Journal of Experimental Medicine, 2023. @article{Tauber#2023,
title = {Landscape of mast cell populations across organs in mice and humans},
author = {Marie Tauber# and Lilian Basso# and Jeremy Martin# and Luciana Bostan and Marlene Magalhaes Pinto and Guilhem R Thierry and Raïssa Houmadi and Nadine Serhan and Alexia Loste and Camille Blériot and Jasper B J Kamphuis and Mirjana Grujic and Lena Kjellén and Gunnar Pejler and Carle Paul and Xinzhong Dong and Stephen J Galli and Laurent L Reber and Florent Ginhoux and Marc Bajenoff and Rebecca Gentek and Nicolas Gaudenzio},
url = {https://pubmed.ncbi.nlm.nih.gov/37462672/},
doi = {10.1084/jem.20230570},
year = {2023},
date = {2023-07-18},
urldate = {2023-07-18},
journal = {Journal of Experimental Medicine},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Scholaert†, Manon; Houmadi†, Raissa; Martin†, Jeremy; Serhan†, Nadine; Tauber, Marie; Braun, Emilie; Basso, Lilian; Merle, Eric; Descargues, Pascal; Viguier, Manuelle; Lesort, Cécile; Chaput, Benoît; Kanitakis, Jean; Jullien, Denis; Livideanu, Cristina Bulai; Lamant, Laurence; Pagès, Emeline; Gaudenzio, Nicolas 3D deconvolution of human skin immune architecture with Multiplex Annotated Tissue Imaging System Article de journal Dans: Science Advances, 2023. @article{Scholaert†2023,
title = {3D deconvolution of human skin immune architecture with Multiplex Annotated Tissue Imaging System},
author = {Manon Scholaert† and Raissa Houmadi† and Jeremy Martin† and Nadine Serhan† and Marie Tauber and Emilie Braun and Lilian Basso and Eric Merle and Pascal Descargues and Manuelle Viguier and Cécile Lesort and Benoît Chaput and Jean Kanitakis and Denis Jullien and Cristina Bulai Livideanu and Laurence Lamant and Emeline Pagès and Nicolas Gaudenzio},
url = {https://www-science-org.proxy.insermbiblio.inist.fr/doi/10.1126/sciadv.adf9491},
doi = {10.1126/sciadv.adf9491},
year = {2023},
date = {2023-06-07},
journal = {Science Advances},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Defaye, Manon; Iftinca, Mircea C.; Gadotti, Vinicius M.; Basso, Lilian; Abdullah, Nasser S.; Cuménal, Mélissa; Agosti, Francina; Hassan, Ahmed; Flynn, Robyn; Martin, Jérémy; Soubeyre, Vanessa; Poulen, Gaetan; Lonjon, Nicolas; Vachiery-Lahaye, Florence; Bauchet, Luc; Mery, Pierre Francois; Bourinet, Emmanuel; Zamponi, Gerald W.; Altier, Christophe The neuronal tyrosine kinase receptor ligand ALKAL2 mediates persistent pain Article de journal Dans: The Journal of Clinical Investigation, 2023. @article{Defaye2023,
title = {The neuronal tyrosine kinase receptor ligand ALKAL2 mediates persistent pain},
author = {Manon Defaye and Mircea C. Iftinca and Vinicius M. Gadotti and Lilian Basso and Nasser S. Abdullah and Mélissa Cuménal and Francina Agosti and Ahmed Hassan and Robyn Flynn and Jérémy Martin and Vanessa Soubeyre and Gaetan Poulen and Nicolas Lonjon and Florence Vachiery-Lahaye and Luc Bauchet and Pierre Francois Mery and Emmanuel Bourinet and Gerald W. Zamponi and Christophe Altier},
url = {https://www-jci-org.proxy.insermbiblio.inist.fr/articles/view/154317},
doi = {https://doi.org/10.1172/JCI154317},
year = {2023},
date = {2023-05-24},
journal = {The Journal of Clinical Investigation},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Starkl, Philipp; Jonsson, Gustav; Artner, Tyler; Turnes, Bruna Lenfers; Serhan, Nadine; Oliveira, Tiago; Gail, Laura-Marie; Stejskal, Karel; Channon, Keith M.; Köcher, Thomas; Stary, Georg; Klang, Victoria; Gaudenzio, Nicolas; Knapp, Sylvia; Woolf, Clifford J.; Penninger, Josef M.; Cronin, Shane J. F. Pre-print | Mast cell-derived BH4 is a critical mediator of postoperative pain Article de journal Dans: bioRxiv, 2023. @article{Starkl2023,
title = {Pre-print | Mast cell-derived BH4 is a critical mediator of postoperative pain},
author = {Philipp Starkl and Gustav Jonsson and Tyler Artner and Bruna Lenfers Turnes and Nadine Serhan and Tiago Oliveira and Laura-Marie Gail and Karel Stejskal and Keith M. Channon and Thomas Köcher and Georg Stary and Victoria Klang and Nicolas Gaudenzio and Sylvia Knapp and Clifford J. Woolf and Josef M. Penninger and Shane J.F. Cronin},
url = {https://www.biorxiv.org/content/10.1101/2023.01.24.525378v1},
year = {2023},
date = {2023-01-24},
journal = {bioRxiv},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2022
|
Nicolas Gaudenzio, Lilian Basso Mast cell-neuron axis in allergy Article de journal Dans: Curr Opin Immunol ., vol. 77, no. 102213, p. 1-6, 2022. @article{Gaudenzio2022,
title = {Mast cell-neuron axis in allergy},
author = {Nicolas Gaudenzio, Lilian Basso},
year = {2022},
date = {2022-05-20},
journal = {Curr Opin Immunol .},
volume = {77},
number = {102213},
pages = {1-6},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Gaudenzio N, Liblau RS. Immune cells impede repair of old neurons Article de journal Dans: Science, vol. 376, no. 6594, p. 694-695, 2022. @article{N2022,
title = {Immune cells impede repair of old neurons},
author = {Gaudenzio N, Liblau RS.},
editor = {{Lambert de Rouvroit}, Catherine},
year = {2022},
date = {2022-05-13},
journal = {Science},
volume = {376},
number = {6594},
pages = { 694-695},
abstract = {The regenerative capacity of older people is reduced, resulting in decreased tissue function and resilience. Accordingly, the regeneration of the sciatic nerve after injury has been reported to be less efficient and slower in older people (1). One of the hallmarks of aging is altered intercellular communication, which is often accompanied by increased density of immune cells within tissues and excessive release of proinflammatory mediators, called inflammaging (2, 3). In this context, the immune system disturbs tissue homeostasis and impedes functional recovery. However, the precise mechanisms underlying this pathophysiological process are largely elusive, which is a barrier to rational treatment design. On page 715 of this issue, Zhou et al. (4) describe a mechanism by which aged sensory neurons release the chemoattractive protein C-X-C motif chemokine ligand 13 (CXCL13). Upon sciatic nerve injury in aged, but not young, mice, this results in the recruitment of CD8+ T cells that prevent axonal regeneration.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The regenerative capacity of older people is reduced, resulting in decreased tissue function and resilience. Accordingly, the regeneration of the sciatic nerve after injury has been reported to be less efficient and slower in older people (1). One of the hallmarks of aging is altered intercellular communication, which is often accompanied by increased density of immune cells within tissues and excessive release of proinflammatory mediators, called inflammaging (2, 3). In this context, the immune system disturbs tissue homeostasis and impedes functional recovery. However, the precise mechanisms underlying this pathophysiological process are largely elusive, which is a barrier to rational treatment design. On page 715 of this issue, Zhou et al. (4) describe a mechanism by which aged sensory neurons release the chemoattractive protein C-X-C motif chemokine ligand 13 (CXCL13). Upon sciatic nerve injury in aged, but not young, mice, this results in the recruitment of CD8+ T cells that prevent axonal regeneration. |
2021
|
Gaudenzio N Stackowicz J, Serhan N Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome Article de journal Dans: J. Exp. Med , vol. 218, no. 10, 2021. @article{J2021,
title = {Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome},
author = {Stackowicz J, Gaudenzio N, Serhan N, Conde E, Godon O, Marichal T, Starkl P, Balbino B, Roers A, Bruhns P, Jönsson F, Moguelet P, Georgin-Lavialle S, Broderick L, Hoffman HM, Galli SJ, Reber},
year = {2021},
date = {2021-09-03},
journal = {J. Exp. Med },
volume = {218},
number = {10},
abstract = {Gain-of-function mutations in NLRP3 are responsible for a spectrum of autoinflammatory diseases collectively referred to as
“cryopyrin-associated periodic syndromes” (CAPS). Treatment of CAPS patients with IL-1–targeted therapies is effective,
confirming a central pathogenic role for IL-1β. However, the specific myeloid cell population(s) exhibiting inflammasome
activity and sustained IL-1β production in CAPS remains elusive. Previous reports suggested an important role for mast cells
(MCs) in this process. Here, we report that, in mice, gain-of-function mutations in Nlrp3 restricted to neutrophils, and to a lesser
extent macrophages/dendritic cells, but not MCs, are sufficient to trigger severe CAPS. Furthermore, in patients with
clinically established CAPS, we show that skin-infiltrating neutrophils represent a substantial biological source of IL-1β.
Together, our data indicate that neutrophils, rather than MCs, can represent the main cellular drivers of CAPS pathology.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Gain-of-function mutations in NLRP3 are responsible for a spectrum of autoinflammatory diseases collectively referred to as
“cryopyrin-associated periodic syndromes” (CAPS). Treatment of CAPS patients with IL-1–targeted therapies is effective,
confirming a central pathogenic role for IL-1β. However, the specific myeloid cell population(s) exhibiting inflammasome
activity and sustained IL-1β production in CAPS remains elusive. Previous reports suggested an important role for mast cells
(MCs) in this process. Here, we report that, in mice, gain-of-function mutations in Nlrp3 restricted to neutrophils, and to a lesser
extent macrophages/dendritic cells, but not MCs, are sufficient to trigger severe CAPS. Furthermore, in patients with
clinically established CAPS, we show that skin-infiltrating neutrophils represent a substantial biological source of IL-1β.
Together, our data indicate that neutrophils, rather than MCs, can represent the main cellular drivers of CAPS pathology. |
Bertrand R Conde E, Balbino B Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice Article de journal Dans: Nature Communications, vol. 12, no. 1, p. 2574, 2021. @article{E2021,
title = {Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice},
author = {Conde E, Bertrand R, Balbino B, Bonnefoy J, Stackowicz J, Caillot N, Colaone F, Hamdi S, Houmadi R, Loste A, Kamphuis JBJ, Huetz F, Guilleminault L, Gaudenzio N, Mougel A, Hardy D, Snouwaert JN, Koller BH, Serra V, Bruhns P, Grouard-Vogel G, Reber LL.},
doi = {10.1038/s41467-021-22834-5},
year = {2021},
date = {2021-05-11},
journal = {Nature Communications},
volume = {12},
number = {1},
pages = {2574},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Starkl, Philipp; Gaudenzio, Nicolas; Marichal, Thomas; Reber, Laurent L; Sibilano, Riccardo; Watzenboeck, Martin L; Fontaine, Frédéric; Mueller, André C; Tsai, Mindy; Knapp, Sylvia; Galli, Stephen J Title: IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells Authors Article de journal Dans: 2021. @article{Starkl,
title = {Title: IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells Authors},
author = {Starkl, Philipp and Gaudenzio, Nicolas and Marichal, Thomas and Reber, Laurent L and Sibilano, Riccardo and Watzenboeck, Martin L and Fontaine, Fr{é}d{é}ric and Mueller, Andr{é} C and Tsai, Mindy and Knapp, Sylvia and Galli, Stephen J},
doi = {10.1111/ALL.14852},
year = {2021},
date = {2021-04-12},
abstract = {Background},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Tauber, Marie; Wang, Fang; Kim, Brian; Gaudenzio, Nicolas; Blank, Ulrich; Kawakami, Toshiaki Bidirectional sensory neuron-immune interactions: a new vision in the understanding of allergic inflammation Article de journal Dans: Current Opinion in Immunology, vol. 72, p. 79–86, 2021. @article{Tauber2021b,
title = {Bidirectional sensory neuron-immune interactions: a new vision in the understanding of allergic inflammation},
author = {Tauber, Marie and Wang, Fang and Kim, Brian and Gaudenzio, Nicolas and Blank, Ulrich and Kawakami, Toshiaki},
url = {https://doi.org/10.1016/j.coi.2021.03.012},
doi = {10.1016/j.coi.2021.03.012},
year = {2021},
date = {2021-01-01},
journal = {Current Opinion in Immunology},
volume = {72},
pages = {79--86},
abstract = {Peripheral neurons (including sensory neurons) are ubiquitously distributed in all tissues, particularly at the interface with the environment. The primary function of sensory neurons is the transmission of sensations of temperature, pain and itch to elicit appropriate behavioral responses. More recently, sensory neurons have emerged as potent regulators of type 2 immune responses and allergic inflammation. There is increasing evidence showing that neurons can express receptors previously thought to be restricted to the immune compartment. In addition, certain subtypes of immune cells (e.g. mast cells, ILC2s or macrophages) also express specific neuroreceptors that provide them with the capacity to integrate neuron-derived signals and modulate their activation status during the development of allergic inflammation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Peripheral neurons (including sensory neurons) are ubiquitously distributed in all tissues, particularly at the interface with the environment. The primary function of sensory neurons is the transmission of sensations of temperature, pain and itch to elicit appropriate behavioral responses. More recently, sensory neurons have emerged as potent regulators of type 2 immune responses and allergic inflammation. There is increasing evidence showing that neurons can express receptors previously thought to be restricted to the immune compartment. In addition, certain subtypes of immune cells (e.g. mast cells, ILC2s or macrophages) also express specific neuroreceptors that provide them with the capacity to integrate neuron-derived signals and modulate their activation status during the development of allergic inflammation. |
Corbière, A.; Loste, A.; Gaudenzio, N. MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases? Article de journal Dans: Exp Dermatol, vol. 30, no. 2, p. 193-200, 2021, (Corbière, Auriane
Loste, Alexia
Gaudenzio, Nicolas
802041/H2020 European Research Council/
Review
Denmark
Exp Dermatol. 2021 Feb;30(2):193-200. doi: 10.1111/exd.14222. Epub 2020 Nov 17.). @article{d,
title = {MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases?},
author = {Corbière, A. and Loste, A. and Gaudenzio, N.},
year = {2021},
date = {2021-01-01},
journal = {Exp Dermatol},
volume = {30},
number = {2},
pages = {193-200},
abstract = {Mast cells are innate immune cells located at many barrier sites in the body and known to protect the host against environmental threats and to be involved in allergic diseases. More recently, new studies have investigated their roles in the regulation of skin inflammation and transmission of pain and itch sensations. Mast cell signalling through the Mas-related G protein-coupled receptor (MRGPR) X2 or its mouse orthologue MRGPRB2 has been reported to be one of the major mechanism by which mast cell can regulate such processes. MRGPRX2 and MRGPRB2 can induce mast cell degranulation upon binding to a broad panel of cationic molecules such as neuropeptides, bacteria-derived quorum sensing molecules, venom peptides, host defense peptides and, unfortunately, various FDA-approved drugs. Upon activation, mast cells release granule-associated proteases, lipids and multiple cytokines that can modulate vascular permeability, immune cells recruitment and activation status of tissue-projecting nociceptive sensory neurons (ie nociceptors). Here, we discuss the modality of MRGPRX2-dependent mast cell activation and its different consequences on the patterns of skin inflammation and associated diseases. We notably emphasize how MRGPRX2-dependent skin mast cell activation might trigger various pathological traits such as pruritus, pain and inflammation and therefore become a potential therapeutic target for inflammatory pain, itch, atopic dermatitis and drugs-induced injection site reactions.},
note = {Corbière, Auriane
Loste, Alexia
Gaudenzio, Nicolas
802041/H2020 European Research Council/
Review
Denmark
Exp Dermatol. 2021 Feb;30(2):193-200. doi: 10.1111/exd.14222. Epub 2020 Nov 17.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells are innate immune cells located at many barrier sites in the body and known to protect the host against environmental threats and to be involved in allergic diseases. More recently, new studies have investigated their roles in the regulation of skin inflammation and transmission of pain and itch sensations. Mast cell signalling through the Mas-related G protein-coupled receptor (MRGPR) X2 or its mouse orthologue MRGPRB2 has been reported to be one of the major mechanism by which mast cell can regulate such processes. MRGPRX2 and MRGPRB2 can induce mast cell degranulation upon binding to a broad panel of cationic molecules such as neuropeptides, bacteria-derived quorum sensing molecules, venom peptides, host defense peptides and, unfortunately, various FDA-approved drugs. Upon activation, mast cells release granule-associated proteases, lipids and multiple cytokines that can modulate vascular permeability, immune cells recruitment and activation status of tissue-projecting nociceptive sensory neurons (ie nociceptors). Here, we discuss the modality of MRGPRX2-dependent mast cell activation and its different consequences on the patterns of skin inflammation and associated diseases. We notably emphasize how MRGPRX2-dependent skin mast cell activation might trigger various pathological traits such as pruritus, pain and inflammation and therefore become a potential therapeutic target for inflammatory pain, itch, atopic dermatitis and drugs-induced injection site reactions. |
Serhan, Nadine; Cenac, Nicolas; Basso, Lilian; Gaudenzio, Nicolas Mas-related G protein-coupled receptors (Mrgprs) - Key regulators of neuroimmune interactions Article de journal Dans: Neuroscience Letters, 2021, ISSN: 03043940. @article{Serhan2021,
title = {Mas-related G protein-coupled receptors (Mrgprs) - Key regulators of neuroimmune interactions},
author = {Serhan, Nadine and Cenac, Nicolas and Basso, Lilian and Gaudenzio, Nicolas},
doi = {10.1016/j.neulet.2021.135724},
issn = {03043940},
year = {2021},
date = {2021-01-01},
journal = {Neuroscience Letters},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Serhan, N.; Cenac, N.; Basso, L.; Gaudenzio, N. Mas-related G protein-coupled receptors (Mrgprs) - Key regulators of neuroimmune interactions Article de journal Dans: Neurosci Lett, vol. 749, p. 135724, 2021, (Serhan, Nadine
Cenac, Nicolas
Basso, Lilian
Gaudenzio, Nicolas
Research Support, Non-U.S. Gov't
Review
Ireland
Neurosci Lett. 2021 Apr 1;749:135724. doi: 10.1016/j.neulet.2021.135724. Epub 2021 Feb 15.). @article{d,
title = {Mas-related G protein-coupled receptors (Mrgprs) - Key regulators of neuroimmune interactions},
author = {Serhan, N. and Cenac, N. and Basso, L. and Gaudenzio, N.},
year = {2021},
date = {2021-01-01},
journal = {Neurosci Lett},
volume = {749},
pages = {135724},
abstract = {Interplay between physiological systems in the body plays a prominent role in health and disease. At the cellular level, such interplay is orchestrated through the binding of specific ligands to their receptors expressed on cell surface. G protein-coupled receptors (GPCR) are seven-transmembrane domain receptors that initiate various cellular responses and regulate homeostasis. In this review, we focus on particular GPCRs named Mas-related G protein-coupled receptors (Mrgprs) mainly expressed by sensory neurons and specialized immune cells. We describe the different subfamilies of Mrgprs and their specific ligands, as well as recent advances in the field that illustrate the role played by these receptors in neuro-immune biological processes, including itch, pain and inflammation in diverse organs.},
note = {Serhan, Nadine
Cenac, Nicolas
Basso, Lilian
Gaudenzio, Nicolas
Research Support, Non-U.S. Gov't
Review
Ireland
Neurosci Lett. 2021 Apr 1;749:135724. doi: 10.1016/j.neulet.2021.135724. Epub 2021 Feb 15.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Interplay between physiological systems in the body plays a prominent role in health and disease. At the cellular level, such interplay is orchestrated through the binding of specific ligands to their receptors expressed on cell surface. G protein-coupled receptors (GPCR) are seven-transmembrane domain receptors that initiate various cellular responses and regulate homeostasis. In this review, we focus on particular GPCRs named Mas-related G protein-coupled receptors (Mrgprs) mainly expressed by sensory neurons and specialized immune cells. We describe the different subfamilies of Mrgprs and their specific ligands, as well as recent advances in the field that illustrate the role played by these receptors in neuro-immune biological processes, including itch, pain and inflammation in diverse organs. |
Starkl, P.; Gaudenzio, N.; Marichal, T.; Reber, L. L.; Sibilano, R.; Watzenboeck, M. L.; Fontaine, F.; Mueller, A. C.; Tsai, M.; Knapp, S.; Galli, S. J. IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells Article de journal Dans: Allergy, 2021, (Starkl, Philipp
Gaudenzio, Nicolas
Marichal, Thomas
Reber, Laurent L
Sibilano, Riccardo
Watzenboeck, Martin L
Fontaine, Frédéric
Mueller, André C
Tsai, Mindy
Knapp, Sylvia
Galli, Stephen J
Fondation Acteria/
J3399-B21/Austrian Science Fund/
P31113-B30/Austrian Science Fund/
655153/H2020 Marie Skłodowska-Curie Actions/
F.4508.18/Fonds De La Recherche Scientifique - FNRS/
Institut National de la Santé et de la Recherche Médicale/
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AI23990/National Institute of Allergy and Infectious Diseases/
R01 AR067145/AR/NIAMS NIH HHS/United States
ANR-18-CE18-0023/Agence Nationale de la Recherche/
ERC-StG-2018 IM-ID #801823/ERC/
ERC-StG-2018 IM-ID #802041/ERC/
Denmark
Allergy. 2021 Apr 11. doi: 10.1111/all.14852.). @article{d,
title = {IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells},
author = {Starkl, P. and Gaudenzio, N. and Marichal, T. and Reber, L. L. and Sibilano, R. and Watzenboeck, M. L. and Fontaine, F. and Mueller, A. C. and Tsai, M. and Knapp, S. and Galli, S. J.},
year = {2021},
date = {2021-01-01},
journal = {Allergy},
abstract = {BACKGROUND: In contrast to their clearly defined roles in allergic diseases, the physiologic functions of Immunoglobulin E antibodies (IgEs) and mast cells (MCs) remain enigmatic. Recent research supports the toxin hypothesis, showing that MCs and IgE-related type 2 immune responses can enhance host defense against certain noxious substances, including honeybee venom (BV). However, the mechanisms by which MCs can interfere with BV toxicity are unknown. In this study, we assessed the role of IgE and certain MC products in MC-mediated BV detoxification. METHODS: We applied in vitro and in vivo fluorescence microscopyimaging, and flow cytometry, fibroblast-based toxicity assays and mass spectrometry to investigate IgE-mediated detoxification of BV cytotoxicity by mouse and human MCs in vitro. Pharmacologic strategies to interfere with MC-derived heparin and proteases helped to define the importance of specific detoxification mechanisms. RESULTS: Venom-specific IgE increased the degranulation and cytokine responses of MCs to BVin vitro. Passive serum sensitization enhanced MC degranulation in vivo. IgE-activated mouse or human MCs exhibited enhanced potential for detoxifying BV by both proteolytic degradation and heparin-related interference with toxicity. Mediators released by IgE-activated human MCs efficiently degraded multiple BV toxins. CONCLUSIONS: Our results both reveal that IgE sensitization enhances the MC's ability to detoxify BV and also assign efficient toxin-neutralizing activity to MC-derived heparin and proteases. Our study thus highlights the potential importance of IgE, MCs, and particular MC products in defense against BV.},
note = {Starkl, Philipp
Gaudenzio, Nicolas
Marichal, Thomas
Reber, Laurent L
Sibilano, Riccardo
Watzenboeck, Martin L
Fontaine, Frédéric
Mueller, André C
Tsai, Mindy
Knapp, Sylvia
Galli, Stephen J
Fondation Acteria/
J3399-B21/Austrian Science Fund/
P31113-B30/Austrian Science Fund/
655153/H2020 Marie Skłodowska-Curie Actions/
F.4508.18/Fonds De La Recherche Scientifique - FNRS/
Institut National de la Santé et de la Recherche Médicale/
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AI23990/National Institute of Allergy and Infectious Diseases/
R01 AR067145/AR/NIAMS NIH HHS/United States
ANR-18-CE18-0023/Agence Nationale de la Recherche/
ERC-StG-2018 IM-ID #801823/ERC/
ERC-StG-2018 IM-ID #802041/ERC/
Denmark
Allergy. 2021 Apr 11. doi: 10.1111/all.14852.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: In contrast to their clearly defined roles in allergic diseases, the physiologic functions of Immunoglobulin E antibodies (IgEs) and mast cells (MCs) remain enigmatic. Recent research supports the toxin hypothesis, showing that MCs and IgE-related type 2 immune responses can enhance host defense against certain noxious substances, including honeybee venom (BV). However, the mechanisms by which MCs can interfere with BV toxicity are unknown. In this study, we assessed the role of IgE and certain MC products in MC-mediated BV detoxification. METHODS: We applied in vitro and in vivo fluorescence microscopyimaging, and flow cytometry, fibroblast-based toxicity assays and mass spectrometry to investigate IgE-mediated detoxification of BV cytotoxicity by mouse and human MCs in vitro. Pharmacologic strategies to interfere with MC-derived heparin and proteases helped to define the importance of specific detoxification mechanisms. RESULTS: Venom-specific IgE increased the degranulation and cytokine responses of MCs to BVin vitro. Passive serum sensitization enhanced MC degranulation in vivo. IgE-activated mouse or human MCs exhibited enhanced potential for detoxifying BV by both proteolytic degradation and heparin-related interference with toxicity. Mediators released by IgE-activated human MCs efficiently degraded multiple BV toxins. CONCLUSIONS: Our results both reveal that IgE sensitization enhances the MC's ability to detoxify BV and also assign efficient toxin-neutralizing activity to MC-derived heparin and proteases. Our study thus highlights the potential importance of IgE, MCs, and particular MC products in defense against BV. |
Tauber, M.; Wang, F.; Kim, B.; Gaudenzio, N. Bidirectional sensory neuron-immune interactions: a new vision in the understanding of allergic inflammation Article de journal Dans: Curr Opin Immunol, vol. 72, p. 79-86, 2021, (Tauber, Marie
Wang, Fang
Kim, Brian
Gaudenzio, Nicolas
Review
England
Curr Opin Immunol. 2021 Apr 16;72:79-86. doi: 10.1016/j.coi.2021.03.012.). @article{d,
title = {Bidirectional sensory neuron-immune interactions: a new vision in the understanding of allergic inflammation},
author = {Tauber, M. and Wang, F. and Kim, B. and Gaudenzio, N.},
year = {2021},
date = {2021-01-01},
journal = {Curr Opin Immunol},
volume = {72},
pages = {79-86},
abstract = {Peripheral neurons (including sensory neurons) are ubiquitously distributed in all tissues, particularly at the interface with the environment. The primary function of sensory neurons is the transmission of sensations of temperature, pain and itch to elicit appropriate behavioral responses. More recently, sensory neurons have emerged as potent regulators of type 2 immune responses and allergic inflammation. There is increasing evidence showing that neurons can express receptors previously thought to be restricted to the immune compartment. In addition, certain subtypes of immune cells (e.g. mast cells, ILC2s or macrophages) also express specific neuroreceptors that provide them with the capacity to integrate neuron-derived signals and modulate their activation status during the development of allergic inflammation.},
note = {Tauber, Marie
Wang, Fang
Kim, Brian
Gaudenzio, Nicolas
Review
England
Curr Opin Immunol. 2021 Apr 16;72:79-86. doi: 10.1016/j.coi.2021.03.012.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Peripheral neurons (including sensory neurons) are ubiquitously distributed in all tissues, particularly at the interface with the environment. The primary function of sensory neurons is the transmission of sensations of temperature, pain and itch to elicit appropriate behavioral responses. More recently, sensory neurons have emerged as potent regulators of type 2 immune responses and allergic inflammation. There is increasing evidence showing that neurons can express receptors previously thought to be restricted to the immune compartment. In addition, certain subtypes of immune cells (e.g. mast cells, ILC2s or macrophages) also express specific neuroreceptors that provide them with the capacity to integrate neuron-derived signals and modulate their activation status during the development of allergic inflammation. |
Conde, Eva; Bertrand, Romain; Balbino, Bianca; Bonnefoy, Jonathan; Stackowicz, Julien; Caillot, Noémie; Colaone, Fabien; Hamdi, Samir; Houmadi, Raïssa; Loste, Alexia; Kamphuis, Jasper B. J.; Huetz, François; Guilleminault, Laurent; Gaudenzio, Nicolas; Mougel, Aurélie; Hardy, David; Snouwaert, John N.; Koller, Beverly H.; Serra, Vincent; Bruhns, Pierre; Grouard-Vogel, Géraldine; Reber, Laurent L. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice Article de journal Dans: Nature Communications, vol. 12, p. 2574, 2021, ISSN: 2041-1723. @article{Conde2021,
title = {Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice},
author = {Eva Conde and Romain Bertrand and Bianca Balbino and Jonathan Bonnefoy and Julien Stackowicz and Noémie Caillot and Fabien Colaone and Samir Hamdi and Raïssa Houmadi and Alexia Loste and Jasper B. J. Kamphuis and François Huetz and Laurent Guilleminault and Nicolas Gaudenzio and Aurélie Mougel and David Hardy and John N. Snouwaert and Beverly H. Koller and Vincent Serra and Pierre Bruhns and Géraldine Grouard-Vogel and Laurent L. Reber},
url = {http://www.nature.com/articles/s41467-021-22834-5},
doi = {10.1038/s41467-021-22834-5},
issn = {2041-1723},
year = {2021},
date = {2021-01-01},
journal = {Nature Communications},
volume = {12},
pages = {2574},
abstract = {<p>Allergic asthma is characterized by elevated levels of IgE antibodies, type 2 cytokines such as interleukin-4 (IL-4) and IL-13, airway hyperresponsiveness (AHR), mucus hypersecretion and eosinophilia. Approved therapeutic monoclonal antibodies targeting IgE or IL-4/IL-13 reduce asthma symptoms but require costly lifelong administrations. Here, we develop conjugate vaccines against mouse IL-4 and IL-13, and demonstrate their prophylactic and therapeutic efficacy in reducing IgE levels, AHR, eosinophilia and mucus production in mouse models of asthma analyzed up to 15 weeks after initial vaccination. More importantly, we also test similar vaccines specific for human IL-4/IL-13 in mice expressing human IL-4/IL-13 and the related receptor, IL-4Rα, to find efficient neutralization of both cytokines and reduced IgE levels for at least 11 weeks post-vaccination. Our results imply that dual IL-4/IL-13 vaccination may represent a cost-effective, long-term therapeutic strategy for the treatment of allergic asthma as demonstrated in mouse models, although additional studies are warranted to assess its safety and feasibility.</p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<p>Allergic asthma is characterized by elevated levels of IgE antibodies, type 2 cytokines such as interleukin-4 (IL-4) and IL-13, airway hyperresponsiveness (AHR), mucus hypersecretion and eosinophilia. Approved therapeutic monoclonal antibodies targeting IgE or IL-4/IL-13 reduce asthma symptoms but require costly lifelong administrations. Here, we develop conjugate vaccines against mouse IL-4 and IL-13, and demonstrate their prophylactic and therapeutic efficacy in reducing IgE levels, AHR, eosinophilia and mucus production in mouse models of asthma analyzed up to 15 weeks after initial vaccination. More importantly, we also test similar vaccines specific for human IL-4/IL-13 in mice expressing human IL-4/IL-13 and the related receptor, IL-4Rα, to find efficient neutralization of both cytokines and reduced IgE levels for at least 11 weeks post-vaccination. Our results imply that dual IL-4/IL-13 vaccination may represent a cost-effective, long-term therapeutic strategy for the treatment of allergic asthma as demonstrated in mouse models, although additional studies are warranted to assess its safety and feasibility.</p> |
2020
|
Starkl, Philipp; Watzenboeck, Martin L; Popov, Lauren M; Zahalka, Sophie; Hladik, Anastasiya; Lakovits, Karin; Radhouani, Mariem; Haschemi, Arvand; Marichal, Thomas; Reber, Laurent L; Gaudenzio, Nicolas; Sibilano, Riccardo; Stulik, Lukas; Fontaine, Frédéric; Mueller, André C; Amieva, Manuel R; Galli, Stephen J; Knapp, Sylvia IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus. Article de journal Dans: Immunity, vol. 53, no. 4, p. 793–804.e9, 2020, ISSN: 1097-4180 (Electronic). @article{Starkl2020,
title = {IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus.},
author = {Starkl, Philipp and Watzenboeck, Martin L and Popov, Lauren M and Zahalka, Sophie and Hladik, Anastasiya and Lakovits, Karin and Radhouani, Mariem and Haschemi, Arvand and Marichal, Thomas and Reber, Laurent L and Gaudenzio, Nicolas and Sibilano, Riccardo and Stulik, Lukas and Fontaine, Fr{é}d{é}ric and Mueller, Andr{é} C and Amieva, Manuel R and Galli, Stephen J and Knapp, Sylvia},
doi = {10.1016/j.immuni.2020.08.002},
issn = {1097-4180 (Electronic)},
year = {2020},
date = {2020-10-01},
journal = {Immunity},
volume = {53},
number = {4},
pages = {793--804.e9},
abstract = {Allergies are considered to represent mal-directed type 2 immune responses against mostly innocuous exogenous compounds. Immunoglobulin E (IgE) antibodies are a characteristic feature of allergies and mediate hypersensitivity against allergens through activation of effector cells, particularly mast cells (MCs). Although the physiological functions of this dangerous branch of immunity have remained enigmatic, recent evidence shows that allergic immune reactions can help to protect against the toxicity of venoms. Because bacteria are a potent alternative source of toxins, we assessed the possible role of allergy-like type 2 immunity in antibacterial host defense. We discovered that the adaptive immune response against Staphylococcus aureus (SA) skin infection substantially improved systemic host defense against secondary SA infections in mice. Moreover, this acquired protection depended on IgE effector mechanisms and MCs. Importantly, our results reveal a previously unknown physiological function of allergic immune responses, IgE antibodies, and MCs in host defense against a pathogenic bacterium.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Allergies are considered to represent mal-directed type 2 immune responses against mostly innocuous exogenous compounds. Immunoglobulin E (IgE) antibodies are a characteristic feature of allergies and mediate hypersensitivity against allergens through activation of effector cells, particularly mast cells (MCs). Although the physiological functions of this dangerous branch of immunity have remained enigmatic, recent evidence shows that allergic immune reactions can help to protect against the toxicity of venoms. Because bacteria are a potent alternative source of toxins, we assessed the possible role of allergy-like type 2 immunity in antibacterial host defense. We discovered that the adaptive immune response against Staphylococcus aureus (SA) skin infection substantially improved systemic host defense against secondary SA infections in mice. Moreover, this acquired protection depended on IgE effector mechanisms and MCs. Importantly, our results reveal a previously unknown physiological function of allergic immune responses, IgE antibodies, and MCs in host defense against a pathogenic bacterium. |
Jendoubi, Fatma; Gaudenzio, Nicolas; Gallini, Adeline; Negretto, Mathilde; Paul, Carle; Bulai Livideanu, Cristina Omalizumab in the treatment of adult patients with mastocytosis: A systematic review. Article de journal Dans: Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, vol. 50, no. 6, p. 654–661, 2020, ISSN: 1365-2222 (Electronic). @article{Jendoubi2020b,
title = {Omalizumab in the treatment of adult patients with mastocytosis: A systematic review.},
author = {Jendoubi, Fatma and Gaudenzio, Nicolas and Gallini, Adeline and Negretto, Mathilde and Paul, Carle and {Bulai Livideanu}, Cristina},
doi = {10.1111/cea.13592},
issn = {1365-2222 (Electronic)},
year = {2020},
date = {2020-06-01},
journal = {Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology},
volume = {50},
number = {6},
pages = {654--661},
abstract = {BACKGROUND: Mastocytosis is associated with mast cell (MC) mediator-related symptoms for which limited therapies are available. OBJECTIVE: Our aim was to assess the efficacy and safety of omalizumab in the treatment of MC mediator-related symptoms in adult patients with mastocytosis. RESULTS: We identified one multi-centre retrospective cohort study (39 patients), one retrospective cohort study (13 patients), 4 case series and 10 case reports. No published controlled randomized study was identified. We included 69 patients (13 patients with cutaneous mastocytosis and 56 with systemic mastocytosis). The mean age was 48 years. Omalizumab maintenance dose was 300 mg for the majority of patients. The mean duration of treatment was 17 months. Treatment led to a tolerability of venom immunotherapy and to a complete resolution of severe reactions in all patients with post-honeybee sting anaphylaxis. Complete resolution of idiopathic anaphylaxis episodes was noted in 84% of the patients. Complete resolution of palpitations, gastrointestinal, cutaneous, neuropsychiatric, respiratory and musculoskeletal symptoms was observed at a rate of 43%, 29%, 27%, 11%, 9% and 0%, respectively. Efficacy was maintained for the entire duration of the treatment in all but four responders. Adverse events were reported for 13 patients. CONCLUSIONS AND CLINICAL RELEVANCE: Omalizumab appears to prevent some life-threatening reactions associated with mastocytosis and may be a good option to treat the associated symptoms. However, the evidence relied upon is observational, uncontrolled and from a small number of patients. A randomized controlled trial is needed to better understand the place of omalizumab in mastocytosis treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Mastocytosis is associated with mast cell (MC) mediator-related symptoms for which limited therapies are available. OBJECTIVE: Our aim was to assess the efficacy and safety of omalizumab in the treatment of MC mediator-related symptoms in adult patients with mastocytosis. RESULTS: We identified one multi-centre retrospective cohort study (39 patients), one retrospective cohort study (13 patients), 4 case series and 10 case reports. No published controlled randomized study was identified. We included 69 patients (13 patients with cutaneous mastocytosis and 56 with systemic mastocytosis). The mean age was 48 years. Omalizumab maintenance dose was 300 mg for the majority of patients. The mean duration of treatment was 17 months. Treatment led to a tolerability of venom immunotherapy and to a complete resolution of severe reactions in all patients with post-honeybee sting anaphylaxis. Complete resolution of idiopathic anaphylaxis episodes was noted in 84% of the patients. Complete resolution of palpitations, gastrointestinal, cutaneous, neuropsychiatric, respiratory and musculoskeletal symptoms was observed at a rate of 43%, 29%, 27%, 11%, 9% and 0%, respectively. Efficacy was maintained for the entire duration of the treatment in all but four responders. Adverse events were reported for 13 patients. CONCLUSIONS AND CLINICAL RELEVANCE: Omalizumab appears to prevent some life-threatening reactions associated with mastocytosis and may be a good option to treat the associated symptoms. However, the evidence relied upon is observational, uncontrolled and from a small number of patients. A randomized controlled trial is needed to better understand the place of omalizumab in mastocytosis treatment. |
Folkerts, Jelle; Gaudenzio, Nicolas; Maurer, Marcus; Hendriks, Rudi W; Stadhouders, Ralph; Tam, See-Ying; Galli, Stephen J Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy. Article de journal Dans: Nature protocols, vol. 15, no. 3, p. 1285–1310, 2020, ISSN: 1750-2799 (Electronic). @article{Folkerts2020,
title = {Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy.},
author = {Folkerts, Jelle and Gaudenzio, Nicolas and Maurer, Marcus and Hendriks, Rudi W and Stadhouders, Ralph and Tam, See-Ying and Galli, Stephen J},
doi = {10.1038/s41596-019-0288-6},
issn = {1750-2799 (Electronic)},
year = {2020},
date = {2020-03-01},
journal = {Nature protocols},
volume = {15},
number = {3},
pages = {1285--1310},
abstract = {Targeted functional genomics represents a powerful approach for studying gene function in vivo and in vitro. However, its application to gene expression studies in human mast cells has been hampered by low yields of human mast cell cultures and their poor transfection efficiency. We developed an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas9 gene editing. By using high-resolution confocal microscopy and a fluorochrome-labeled avidin probe, one can directly assess the alteration of functional responses, i.e., degranulation, in single human mast cells (10-12 weeks old). The elimination of a drug or marker selection step avoids the use of potentially toxic treatment procedures, and the brief hands-on time of the functional analysis step enables high-throughput screening of shRNA or CRISPR-Cas9 constructs to identify genes that regulate human mast cell degranulation. The ability to analyze single cells substantially reduces the total number of cells required and enables the parallel visualization of the degranulation profiles of both edited and non-edited mast cells, offering a consistent internal control not found in other protocols. Moreover, our protocol offers a flexible choice between RNA interference (RNAi) and CRISPR-Cas9 genome editing for perturbation of gene expression using our human mast cell single-cell imaging system. Perturbation of gene expression, acquisition of microscopy data and image analysis can be completed within 5 d, requiring only standard laboratory equipment and expertise.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Targeted functional genomics represents a powerful approach for studying gene function in vivo and in vitro. However, its application to gene expression studies in human mast cells has been hampered by low yields of human mast cell cultures and their poor transfection efficiency. We developed an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas9 gene editing. By using high-resolution confocal microscopy and a fluorochrome-labeled avidin probe, one can directly assess the alteration of functional responses, i.e., degranulation, in single human mast cells (10-12 weeks old). The elimination of a drug or marker selection step avoids the use of potentially toxic treatment procedures, and the brief hands-on time of the functional analysis step enables high-throughput screening of shRNA or CRISPR-Cas9 constructs to identify genes that regulate human mast cell degranulation. The ability to analyze single cells substantially reduces the total number of cells required and enables the parallel visualization of the degranulation profiles of both edited and non-edited mast cells, offering a consistent internal control not found in other protocols. Moreover, our protocol offers a flexible choice between RNA interference (RNAi) and CRISPR-Cas9 genome editing for perturbation of gene expression using our human mast cell single-cell imaging system. Perturbation of gene expression, acquisition of microscopy data and image analysis can be completed within 5 d, requiring only standard laboratory equipment and expertise. |
Meixiong, James; Basso, Lilian; Dong, Xinzhong; Gaudenzio, Nicolas Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis. Article de journal Dans: Trends in neurosciences, vol. 43, no. 3, p. 130–132, 2020, ISSN: 1878-108X (Electronic). @article{Meixiong2020,
title = {Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis.},
author = {Meixiong, James and Basso, Lilian and Dong, Xinzhong and Gaudenzio, Nicolas},
doi = {10.1016/j.tins.2020.01.001},
issn = {1878-108X (Electronic)},
year = {2020},
date = {2020-03-01},
journal = {Trends in neurosciences},
volume = {43},
number = {3},
pages = {130--132},
abstract = {Recent studies revealed the existence of unique functional links between mast cells and nociceptors in the skin. Here, we propose that mast cells and nociceptors form a single regulatory unit in both physiology and disease. In this model, MrgprB2/X2 signaling is a primary mechanism by which mast cells functionally interact with nociceptors to form specialized neuroimmune clusters that regulate pain, inflammation, and itch.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Recent studies revealed the existence of unique functional links between mast cells and nociceptors in the skin. Here, we propose that mast cells and nociceptors form a single regulatory unit in both physiology and disease. In this model, MrgprB2/X2 signaling is a primary mechanism by which mast cells functionally interact with nociceptors to form specialized neuroimmune clusters that regulate pain, inflammation, and itch. |
Corbière, Auriane; Loste, Alexia; Gaudenzio, Nicolas MRGPRX2 sensing of cationic compounds—A bridge between nociception and skin diseases? Article de journal Dans: Experimental Dermatology, 2020, ISSN: 16000625. @article{Corbiere2020,
title = {MRGPRX2 sensing of cationic compounds—A bridge between nociception and skin diseases?},
author = {Corbi{è}re, Auriane and Loste, Alexia and Gaudenzio, Nicolas},
doi = {10.1111/exd.14222},
issn = {16000625},
year = {2020},
date = {2020-01-01},
journal = {Experimental Dermatology},
abstract = {Mast cells are innate immune cells located at many barrier sites in the body and known to protect the host against environmental threats and to be involved in allergic diseases. More recently, new studies have investigated their roles in the regulation of skin inflammation and transmission of pain and itch sensations. Mast cell signalling through the Mas-related G protein-coupled receptor (MRGPR) X2 or its mouse orthologue MRGPRB2 has been reported to be one of the major mechanism by which mast cell can regulate such processes. MRGPRX2 and MRGPRB2 can induce mast cell degranulation upon binding to a broad panel of cationic molecules such as neuropeptides, bacteria-derived quorum sensing molecules, venom peptides, host defense peptides and, unfortunately, various FDA-approved drugs. Upon activation, mast cells release granule-associated proteases, lipids and multiple cytokines that can modulate vascular permeability, immune cells recruitment and activation status of tissue-projecting nociceptive sensory neurons (ie nociceptors). Here, we discuss the modality of MRGPRX2-dependent mast cell activation and its different consequences on the patterns of skin inflammation and associated diseases. We notably emphasize how MRGPRX2-dependent skin mast cell activation might trigger various pathological traits such as pruritus, pain and inflammation and therefore become a potential therapeutic target for inflammatory pain, itch, atopic dermatitis and drugs-induced injection site reactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells are innate immune cells located at many barrier sites in the body and known to protect the host against environmental threats and to be involved in allergic diseases. More recently, new studies have investigated their roles in the regulation of skin inflammation and transmission of pain and itch sensations. Mast cell signalling through the Mas-related G protein-coupled receptor (MRGPR) X2 or its mouse orthologue MRGPRB2 has been reported to be one of the major mechanism by which mast cell can regulate such processes. MRGPRX2 and MRGPRB2 can induce mast cell degranulation upon binding to a broad panel of cationic molecules such as neuropeptides, bacteria-derived quorum sensing molecules, venom peptides, host defense peptides and, unfortunately, various FDA-approved drugs. Upon activation, mast cells release granule-associated proteases, lipids and multiple cytokines that can modulate vascular permeability, immune cells recruitment and activation status of tissue-projecting nociceptive sensory neurons (ie nociceptors). Here, we discuss the modality of MRGPRX2-dependent mast cell activation and its different consequences on the patterns of skin inflammation and associated diseases. We notably emphasize how MRGPRX2-dependent skin mast cell activation might trigger various pathological traits such as pruritus, pain and inflammation and therefore become a potential therapeutic target for inflammatory pain, itch, atopic dermatitis and drugs-induced injection site reactions. |
Starkl, P.; Watzenboeck, M. L.; Popov, L. M.; Zahalka, S.; Hladik, A.; Lakovits, K.; Radhouani, M.; Haschemi, A.; Marichal, T.; Reber, L. L.; Gaudenzio, N.; Sibilano, R.; Stulik, L.; Fontaine, F.; Mueller, A. C.; Amieva, M. R.; Galli, S. J.; Knapp, S. IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus Article de journal Dans: Immunity, vol. 53, no. 4, p. 793-804 e9, 2020, (Starkl, Philipp
Watzenboeck, Martin L
Popov, Lauren M
Zahalka, Sophie
Hladik, Anastasiya
Lakovits, Karin
Radhouani, Mariem
Haschemi, Arvand
Marichal, Thomas
Reber, Laurent L
Gaudenzio, Nicolas
Sibilano, Riccardo
Stulik, Lukas
Fontaine, Frédéric
Mueller, André C
Amieva, Manuel R
Galli, Stephen J
Knapp, Sylvia
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Immunity. 2020 Oct 13;53(4):793-804.e9. doi: 10.1016/j.immuni.2020.08.002. Epub 2020 Sep 9.). @article{d,
title = {IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus},
author = {Starkl, P. and Watzenboeck, M. L. and Popov, L. M. and Zahalka, S. and Hladik, A. and Lakovits, K. and Radhouani, M. and Haschemi, A. and Marichal, T. and Reber, L. L. and Gaudenzio, N. and Sibilano, R. and Stulik, L. and Fontaine, F. and Mueller, A. C. and Amieva, M. R. and Galli, S. J. and Knapp, S.},
year = {2020},
date = {2020-01-01},
journal = {Immunity},
volume = {53},
number = {4},
pages = {793-804 e9},
abstract = {Allergies are considered to represent mal-directed type 2 immune responses against mostly innocuous exogenous compounds. Immunoglobulin E (IgE) antibodies are a characteristic feature of allergies and mediate hypersensitivity against allergens through activation of effector cells, particularly mast cells (MCs). Although the physiological functions of this dangerous branch of immunity have remained enigmatic, recent evidence shows that allergic immune reactions can help to protect against the toxicity of venoms. Because bacteria are a potent alternative source of toxins, we assessed the possible role of allergy-like type 2 immunity in antibacterial host defense. We discovered that the adaptive immune response against Staphylococcus aureus (SA) skin infection substantially improved systemic host defense against secondary SA infections in mice. Moreover, this acquired protection depended on IgE effector mechanisms and MCs. Importantly, our results reveal a previously unknown physiological function of allergic immune responses, IgE antibodies, and MCs in host defense against a pathogenic bacterium.},
note = {Starkl, Philipp
Watzenboeck, Martin L
Popov, Lauren M
Zahalka, Sophie
Hladik, Anastasiya
Lakovits, Karin
Radhouani, Mariem
Haschemi, Arvand
Marichal, Thomas
Reber, Laurent L
Gaudenzio, Nicolas
Sibilano, Riccardo
Stulik, Lukas
Fontaine, Frédéric
Mueller, André C
Amieva, Manuel R
Galli, Stephen J
Knapp, Sylvia
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Immunity. 2020 Oct 13;53(4):793-804.e9. doi: 10.1016/j.immuni.2020.08.002. Epub 2020 Sep 9.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Allergies are considered to represent mal-directed type 2 immune responses against mostly innocuous exogenous compounds. Immunoglobulin E (IgE) antibodies are a characteristic feature of allergies and mediate hypersensitivity against allergens through activation of effector cells, particularly mast cells (MCs). Although the physiological functions of this dangerous branch of immunity have remained enigmatic, recent evidence shows that allergic immune reactions can help to protect against the toxicity of venoms. Because bacteria are a potent alternative source of toxins, we assessed the possible role of allergy-like type 2 immunity in antibacterial host defense. We discovered that the adaptive immune response against Staphylococcus aureus (SA) skin infection substantially improved systemic host defense against secondary SA infections in mice. Moreover, this acquired protection depended on IgE effector mechanisms and MCs. Importantly, our results reveal a previously unknown physiological function of allergic immune responses, IgE antibodies, and MCs in host defense against a pathogenic bacterium. |
Meixiong, J.; Basso, L.; Dong, X.; Gaudenzio, N. Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis Article de journal Dans: Trends Neurosci, vol. 43, no. 3, p. 130-132, 2020, (Meixiong, James
Basso, Lilian
Dong, Xinzhong
Gaudenzio, Nicolas
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
England
Trends Neurosci. 2020 Mar;43(3):130-132. doi: 10.1016/j.tins.2020.01.001. Epub 2020 Jan 31.). @article{d,
title = {Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis},
author = {Meixiong, J. and Basso, L. and Dong, X. and Gaudenzio, N.},
year = {2020},
date = {2020-01-01},
journal = {Trends Neurosci},
volume = {43},
number = {3},
pages = {130-132},
abstract = {Recent studies revealed the existence of unique functional links between mast cells and nociceptors in the skin. Here, we propose that mast cells and nociceptors form a single regulatory unit in both physiology and disease. In this model, MrgprB2/X2 signaling is a primary mechanism by which mast cells functionally interact with nociceptors to form specialized neuroimmune clusters that regulate pain, inflammation, and itch.},
note = {Meixiong, James
Basso, Lilian
Dong, Xinzhong
Gaudenzio, Nicolas
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
England
Trends Neurosci. 2020 Mar;43(3):130-132. doi: 10.1016/j.tins.2020.01.001. Epub 2020 Jan 31.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Recent studies revealed the existence of unique functional links between mast cells and nociceptors in the skin. Here, we propose that mast cells and nociceptors form a single regulatory unit in both physiology and disease. In this model, MrgprB2/X2 signaling is a primary mechanism by which mast cells functionally interact with nociceptors to form specialized neuroimmune clusters that regulate pain, inflammation, and itch. |
Jendoubi, F.; Gaudenzio, N.; Gallini, A.; Negretto, M.; Paul, C.; Bulai Livideanu, C. Omalizumab in the treatment of adult patients with mastocytosis: A systematic review Article de journal Dans: Clin Exp Allergy, vol. 50, no. 6, p. 654-661, 2020, (Jendoubi, Fatma
Gaudenzio, Nicolas
Gallini, Adeline
Negretto, Mathilde
Paul, Carle
Bulai Livideanu, Cristina
England
Clin Exp Allergy. 2020 Jun;50(6):654-661. doi: 10.1111/cea.13592. Epub 2020 Mar 25.). @article{d,
title = {Omalizumab in the treatment of adult patients with mastocytosis: A systematic review},
author = {Jendoubi, F. and Gaudenzio, N. and Gallini, A. and Negretto, M. and Paul, C. and Bulai Livideanu, C.},
year = {2020},
date = {2020-01-01},
journal = {Clin Exp Allergy},
volume = {50},
number = {6},
pages = {654-661},
abstract = {BACKGROUND: Mastocytosis is associated with mast cell (MC) mediator-related symptoms for which limited therapies are available. OBJECTIVE: Our aim was to assess the efficacy and safety of omalizumab in the treatment of MC mediator-related symptoms in adult patients with mastocytosis. RESULTS: We identified one multi-centre retrospective cohort study (39 patients), one retrospective cohort study (13 patients), 4 case series and 10 case reports. No published controlled randomized study was identified. We included 69 patients (13 patients with cutaneous mastocytosis and 56 with systemic mastocytosis). The mean age was 48 years. Omalizumab maintenance dose was 300 mg for the majority of patients. The mean duration of treatment was 17 months. Treatment led to a tolerability of venom immunotherapy and to a complete resolution of severe reactions in all patients with post-honeybee sting anaphylaxis. Complete resolution of idiopathic anaphylaxis episodes was noted in 84% of the patients. Complete resolution of palpitations, gastrointestinal, cutaneous, neuropsychiatric, respiratory and musculoskeletal symptoms was observed at a rate of 43%, 29%, 27%, 11%, 9% and 0%, respectively. Efficacy was maintained for the entire duration of the treatment in all but four responders. Adverse events were reported for 13 patients. CONCLUSIONS AND CLINICAL RELEVANCE: Omalizumab appears to prevent some life-threatening reactions associated with mastocytosis and may be a good option to treat the associated symptoms. However, the evidence relied upon is observational, uncontrolled and from a small number of patients. A randomized controlled trial is needed to better understand the place of omalizumab in mastocytosis treatment.},
note = {Jendoubi, Fatma
Gaudenzio, Nicolas
Gallini, Adeline
Negretto, Mathilde
Paul, Carle
Bulai Livideanu, Cristina
England
Clin Exp Allergy. 2020 Jun;50(6):654-661. doi: 10.1111/cea.13592. Epub 2020 Mar 25.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Mastocytosis is associated with mast cell (MC) mediator-related symptoms for which limited therapies are available. OBJECTIVE: Our aim was to assess the efficacy and safety of omalizumab in the treatment of MC mediator-related symptoms in adult patients with mastocytosis. RESULTS: We identified one multi-centre retrospective cohort study (39 patients), one retrospective cohort study (13 patients), 4 case series and 10 case reports. No published controlled randomized study was identified. We included 69 patients (13 patients with cutaneous mastocytosis and 56 with systemic mastocytosis). The mean age was 48 years. Omalizumab maintenance dose was 300 mg for the majority of patients. The mean duration of treatment was 17 months. Treatment led to a tolerability of venom immunotherapy and to a complete resolution of severe reactions in all patients with post-honeybee sting anaphylaxis. Complete resolution of idiopathic anaphylaxis episodes was noted in 84% of the patients. Complete resolution of palpitations, gastrointestinal, cutaneous, neuropsychiatric, respiratory and musculoskeletal symptoms was observed at a rate of 43%, 29%, 27%, 11%, 9% and 0%, respectively. Efficacy was maintained for the entire duration of the treatment in all but four responders. Adverse events were reported for 13 patients. CONCLUSIONS AND CLINICAL RELEVANCE: Omalizumab appears to prevent some life-threatening reactions associated with mastocytosis and may be a good option to treat the associated symptoms. However, the evidence relied upon is observational, uncontrolled and from a small number of patients. A randomized controlled trial is needed to better understand the place of omalizumab in mastocytosis treatment. |
Galli, S. J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns Article de journal Dans: Annu Rev Immunol, vol. 38, p. 49-77, 2020, (Galli, Stephen J
Gaudenzio, Nicolas
Tsai, Mindy
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
United States
Annu Rev Immunol. 2020 Apr 26;38:49-77. doi: 10.1146/annurev-immunol-071719-094903.). @article{d,
title = {Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns},
author = {Galli, S. J. and Gaudenzio, N. and Tsai, M.},
year = {2020},
date = {2020-01-01},
journal = {Annu Rev Immunol},
volume = {38},
pages = {49-77},
abstract = {Mast cells have existed long before the development of adaptive immunity, although they have been given different names. Thus, in the marine urochordate Styela plicata, they have been designated as test cells. However, based on their morphological characteristics (including prominent cytoplasmic granules) and mediator content (including heparin, histamine, and neutral proteases), test cells are thought to represent members of the lineage known in vertebrates as mast cells. So this lineage presumably had important functions that preceded the development of antibodies, including IgE. Yet mast cells are best known, in humans, as key sources of mediators responsible for acute allergic reactions, notably including anaphylaxis, a severe and potentially fatal IgE-dependent immediate hypersensitivity reaction to apparently harmless antigens, including many found in foods and medicines. In this review, we briefly describe the origins of tissue mast cells and outline evidence that these cells can have beneficial as well as detrimental functions, both innately and as participants in adaptive immune responses. We also discuss aspects of mast cell heterogeneity and comment on how the plasticity of this lineage may provide insight into its roles in health and disease. Finally, we consider some currently open questions that are yet unresolved.},
note = {Galli, Stephen J
Gaudenzio, Nicolas
Tsai, Mindy
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
United States
Annu Rev Immunol. 2020 Apr 26;38:49-77. doi: 10.1146/annurev-immunol-071719-094903.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells have existed long before the development of adaptive immunity, although they have been given different names. Thus, in the marine urochordate Styela plicata, they have been designated as test cells. However, based on their morphological characteristics (including prominent cytoplasmic granules) and mediator content (including heparin, histamine, and neutral proteases), test cells are thought to represent members of the lineage known in vertebrates as mast cells. So this lineage presumably had important functions that preceded the development of antibodies, including IgE. Yet mast cells are best known, in humans, as key sources of mediators responsible for acute allergic reactions, notably including anaphylaxis, a severe and potentially fatal IgE-dependent immediate hypersensitivity reaction to apparently harmless antigens, including many found in foods and medicines. In this review, we briefly describe the origins of tissue mast cells and outline evidence that these cells can have beneficial as well as detrimental functions, both innately and as participants in adaptive immune responses. We also discuss aspects of mast cell heterogeneity and comment on how the plasticity of this lineage may provide insight into its roles in health and disease. Finally, we consider some currently open questions that are yet unresolved. |
Folkerts, J.; Gaudenzio, N.; Maurer, M.; Hendriks, R. W.; Stadhouders, R.; Tam, S. Y.; Galli, S. J. Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy Article de journal Dans: Nat Protoc, vol. 15, no. 3, p. 1285-1310, 2020, (Folkerts, Jelle
Gaudenzio, Nicolas
Maurer, Marcus
Hendriks, Rudi W
Stadhouders, Ralph
Tam, See-Ying
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Protoc. 2020 Mar;15(3):1285-1310. doi: 10.1038/s41596-019-0288-6. Epub 2020 Feb 14.). @article{d,
title = {Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy},
author = {Folkerts, J. and Gaudenzio, N. and Maurer, M. and Hendriks, R. W. and Stadhouders, R. and Tam, S. Y. and Galli, S. J.},
year = {2020},
date = {2020-01-01},
journal = {Nat Protoc},
volume = {15},
number = {3},
pages = {1285-1310},
abstract = {Targeted functional genomics represents a powerful approach for studying gene function in vivo and in vitro. However, its application to gene expression studies in human mast cells has been hampered by low yields of human mast cell cultures and their poor transfection efficiency. We developed an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas9 gene editing. By using high-resolution confocal microscopy and a fluorochrome-labeled avidin probe, one can directly assess the alteration of functional responses, i.e., degranulation, in single human mast cells (10-12 weeks old). The elimination of a drug or marker selection step avoids the use of potentially toxic treatment procedures, and the brief hands-on time of the functional analysis step enables high-throughput screening of shRNA or CRISPR-Cas9 constructs to identify genes that regulate human mast cell degranulation. The ability to analyze single cells substantially reduces the total number of cells required and enables the parallel visualization of the degranulation profiles of both edited and non-edited mast cells, offering a consistent internal control not found in other protocols. Moreover, our protocol offers a flexible choice between RNA interference (RNAi) and CRISPR-Cas9 genome editing for perturbation of gene expression using our human mast cell single-cell imaging system. Perturbation of gene expression, acquisition of microscopy data and image analysis can be completed within 5 d, requiring only standard laboratory equipment and expertise.},
note = {Folkerts, Jelle
Gaudenzio, Nicolas
Maurer, Marcus
Hendriks, Rudi W
Stadhouders, Ralph
Tam, See-Ying
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Protoc. 2020 Mar;15(3):1285-1310. doi: 10.1038/s41596-019-0288-6. Epub 2020 Feb 14.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Targeted functional genomics represents a powerful approach for studying gene function in vivo and in vitro. However, its application to gene expression studies in human mast cells has been hampered by low yields of human mast cell cultures and their poor transfection efficiency. We developed an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas9 gene editing. By using high-resolution confocal microscopy and a fluorochrome-labeled avidin probe, one can directly assess the alteration of functional responses, i.e., degranulation, in single human mast cells (10-12 weeks old). The elimination of a drug or marker selection step avoids the use of potentially toxic treatment procedures, and the brief hands-on time of the functional analysis step enables high-throughput screening of shRNA or CRISPR-Cas9 constructs to identify genes that regulate human mast cell degranulation. The ability to analyze single cells substantially reduces the total number of cells required and enables the parallel visualization of the degranulation profiles of both edited and non-edited mast cells, offering a consistent internal control not found in other protocols. Moreover, our protocol offers a flexible choice between RNA interference (RNAi) and CRISPR-Cas9 genome editing for perturbation of gene expression using our human mast cell single-cell imaging system. Perturbation of gene expression, acquisition of microscopy data and image analysis can be completed within 5 d, requiring only standard laboratory equipment and expertise. |
Arock, M.; Blank, U.; Charles, N.; Gaudenzio, N.; Georgin-Lavialle, S.; Li, M.; Ménasché, G.; Reber, L.; Vitte, J. The "Mast Cell and Basophil Club" of the French Society for Immunology Article de journal Dans: Eur J Immunol, vol. 50, no. 10, p. 1430-1431, 2020, (Arock, Michel
Blank, Ulrich
Charles, Nicolas
Gaudenzio, Nicolas
Georgin-Lavialle, Sophie
Li, Mei
Ménasché, Gaël
Reber, Laurent
Vitte, Joana
Germany
Eur J Immunol. 2020 Oct;50(10):1430-1431. doi: 10.1002/eji.202070105.). @article{d,
title = {The "Mast Cell and Basophil Club" of the French Society for Immunology},
author = {Arock, M. and Blank, U. and Charles, N. and Gaudenzio, N. and Georgin-Lavialle, S. and Li, M. and Ménasché, G. and Reber, L. and Vitte, J.},
year = {2020},
date = {2020-01-01},
journal = {Eur J Immunol},
volume = {50},
number = {10},
pages = {1430-1431},
note = {Arock, Michel
Blank, Ulrich
Charles, Nicolas
Gaudenzio, Nicolas
Georgin-Lavialle, Sophie
Li, Mei
Ménasché, Gaël
Reber, Laurent
Vitte, Joana
Germany
Eur J Immunol. 2020 Oct;50(10):1430-1431. doi: 10.1002/eji.202070105.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Galli, Stephen J; Gaudenzio, Nicolas; Tsai, Mindy Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns Article de journal Dans: Annual Review of Immunology, vol. 5, 2020. @article{Galli2020,
title = {Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns},
author = {Stephen J Galli and Nicolas Gaudenzio and Mindy Tsai},
url = {https://doi.org/10.1146/annurev-immunol-071719-},
doi = {10.1146/annurev-immunol-071719},
year = {2020},
date = {2020-01-01},
journal = {Annual Review of Immunology},
volume = {5},
abstract = {Mast cells have existed long before the development of adaptive immunity, although they have been given different names. Thus, in the marine urochor-date Styela plicata, they have been designated as test cells. However, based on their morphological characteristics (including prominent cytoplasmic granules) and mediator content (including heparin, histamine, and neutral pro-teases), test cells are thought to represent members of the lineage known in vertebrates as mast cells. So this lineage presumably had important functions that preceded the development of antibodies, including IgE. Yet mast cells are best known, in humans, as key sources of mediators responsible for acute allergic reactions, notably including anaphylaxis, a severe and potentially fatal IgE-dependent immediate hypersensitivity reaction to apparently harmless antigens, including many found in foods and medicines. In this review, we briefly describe the origins of tissue mast cells and outline evidence that 49},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells have existed long before the development of adaptive immunity, although they have been given different names. Thus, in the marine urochor-date Styela plicata, they have been designated as test cells. However, based on their morphological characteristics (including prominent cytoplasmic granules) and mediator content (including heparin, histamine, and neutral pro-teases), test cells are thought to represent members of the lineage known in vertebrates as mast cells. So this lineage presumably had important functions that preceded the development of antibodies, including IgE. Yet mast cells are best known, in humans, as key sources of mediators responsible for acute allergic reactions, notably including anaphylaxis, a severe and potentially fatal IgE-dependent immediate hypersensitivity reaction to apparently harmless antigens, including many found in foods and medicines. In this review, we briefly describe the origins of tissue mast cells and outline evidence that 49 |
2019
|
Basso, Lilian; Serhan, Nadine; Tauber, Marie; Gaudenzio, Nicolas Peripheral neurons: Master regulators of skin and mucosal immune response. Article de journal Dans: European journal of immunology, vol. 49, no. 11, p. 1984–1997, 2019, ISSN: 1521-4141 (Electronic). @article{Basso2019,
title = {Peripheral neurons: Master regulators of skin and mucosal immune response.},
author = {Basso, Lilian and Serhan, Nadine and Tauber, Marie and Gaudenzio, Nicolas},
doi = {10.1002/eji.201848027},
issn = {1521-4141 (Electronic)},
year = {2019},
date = {2019-11-01},
journal = {European journal of immunology},
volume = {49},
number = {11},
pages = {1984--1997},
abstract = {The body is innervated by a meshwork of heterogeneous peripheral neurons (including sensory neurons) which project virtually to all the organs. Peripheral neurons have been studied extensively in the context of their primary function of initiation of voluntary and involuntary movement, transmission of sensations and induction of appropriate behavioral response such as withdrawal to avoid tissue injury or scratching to remove irritating molecules. More recently, breakthrough articles have shown that, on top of their primary function of signal transmission to the spinal cord and brain, peripheral neurons (including afferent neurons) could directly sense environmental alarms and consequently regulate the development of various type of immune responses through the release of neuropeptides or growth factors. In this review, we discuss recent advances in the neural regulation of the immune response, both in physiological and pathological contexts by taking into account the type of organs (lungs, skin and gut), subtypes of peripheral neurons (sympathetic, nociceptive and intrinsic gut neurons) or immune cells and strains of pathogens studied. We also highlight future challenges in the field and potential therapeutic innovations targeting neuro-immune interactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The body is innervated by a meshwork of heterogeneous peripheral neurons (including sensory neurons) which project virtually to all the organs. Peripheral neurons have been studied extensively in the context of their primary function of initiation of voluntary and involuntary movement, transmission of sensations and induction of appropriate behavioral response such as withdrawal to avoid tissue injury or scratching to remove irritating molecules. More recently, breakthrough articles have shown that, on top of their primary function of signal transmission to the spinal cord and brain, peripheral neurons (including afferent neurons) could directly sense environmental alarms and consequently regulate the development of various type of immune responses through the release of neuropeptides or growth factors. In this review, we discuss recent advances in the neural regulation of the immune response, both in physiological and pathological contexts by taking into account the type of organs (lungs, skin and gut), subtypes of peripheral neurons (sympathetic, nociceptive and intrinsic gut neurons) or immune cells and strains of pathogens studied. We also highlight future challenges in the field and potential therapeutic innovations targeting neuro-immune interactions. |
Pundir, Priyanka; Liu, Rui; Vasavda, Chirag; Serhan, Nadine; Limjunyawong, Nathachit; Yee, Rebecca; Zhan, Yingzhuan; Dong, Xintong; Wu, Xueqing; Zhang, Ying; Snyder, Solomon H; Gaudenzio, Nicolas; Vidal, Jorge E; Dong, Xinzhong A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Article de journal Dans: Cell host & microbe, vol. 26, no. 1, p. 114–122.e8, 2019, ISSN: 1934-6069 (Electronic). @article{Pundir2019,
title = {A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity.},
author = {Pundir, Priyanka and Liu, Rui and Vasavda, Chirag and Serhan, Nadine and Limjunyawong, Nathachit and Yee, Rebecca and Zhan, Yingzhuan and Dong, Xintong and Wu, Xueqing and Zhang, Ying and Snyder, Solomon H and Gaudenzio, Nicolas and Vidal, Jorge E and Dong, Xinzhong},
doi = {10.1016/j.chom.2019.06.003},
issn = {1934-6069 (Electronic)},
year = {2019},
date = {2019-07-01},
journal = {Cell host & microbe},
volume = {26},
number = {1},
pages = {114--122.e8},
abstract = {Quorum-sensing molecules (QSMs) are secreted by bacteria to signal population density. Upon reaching a critical concentration, QSMs induce transcriptional alterations in bacteria, which enable virulence factor expression and biofilm formation. It is unclear whether mammalian hosts can recognize QSMs to trigger responsive antibacterial immunity. We report that mouse mast-cell-specific G-protein-coupled receptor Mrgprb2 and its human homolog MRGPRX2 are receptors for Gram-positive QSMs, including competence-stimulating peptide (CSP)-1. CSP-1 activates Mrgprb2 and MRGPRX2, triggering mast cell degranulation, which inhibits bacterial growth and prevents biofilm formation. Such antibacterial functions are reduced in Mrgprb2-deficient mast cells, while wild-type mast cells fail to inhibit the growth of bacterial strains lacking CSP-1. Mrgprb2-knockout mice exhibit reduced bacterial clearance, while pharmacologically activating Mrgprb2 in vivo eliminates bacteria and improves disease score. These findings identify a host defense mechanism that uses QSMs as an "Achilles heel" and suggest MRGPRX2 as a potential therapeutic target for controlling bacterial infections.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Quorum-sensing molecules (QSMs) are secreted by bacteria to signal population density. Upon reaching a critical concentration, QSMs induce transcriptional alterations in bacteria, which enable virulence factor expression and biofilm formation. It is unclear whether mammalian hosts can recognize QSMs to trigger responsive antibacterial immunity. We report that mouse mast-cell-specific G-protein-coupled receptor Mrgprb2 and its human homolog MRGPRX2 are receptors for Gram-positive QSMs, including competence-stimulating peptide (CSP)-1. CSP-1 activates Mrgprb2 and MRGPRX2, triggering mast cell degranulation, which inhibits bacterial growth and prevents biofilm formation. Such antibacterial functions are reduced in Mrgprb2-deficient mast cells, while wild-type mast cells fail to inhibit the growth of bacterial strains lacking CSP-1. Mrgprb2-knockout mice exhibit reduced bacterial clearance, while pharmacologically activating Mrgprb2 in vivo eliminates bacteria and improves disease score. These findings identify a host defense mechanism that uses QSMs as an "Achilles heel" and suggest MRGPRX2 as a potential therapeutic target for controlling bacterial infections. |
Serhan, Nadine; Basso, Lilian; Sibilano, Riccardo; Petitfils, Camille; Meixiong, James; Bonnart, Chrystelle; Reber, Laurent L.; Marichal, Thomas; Starkl, Philipp; Cenac, Nicolas; Dong, Xinzhong; Tsai, Mindy; Galli, Stephen J.; Gaudenzio, Nicolas House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation Article de journal Dans: Nature Immunology, 2019, ISSN: 15292916. @article{Serhan2019,
title = {House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation},
author = {Serhan, Nadine and Basso, Lilian and Sibilano, Riccardo and Petitfils, Camille and Meixiong, James and Bonnart, Chrystelle and Reber, Laurent L. and Marichal, Thomas and Starkl, Philipp and Cenac, Nicolas and Dong, Xinzhong and Tsai, Mindy and Galli, Stephen J. and Gaudenzio, Nicolas},
doi = {10.1038/s41590-019-0493-z},
issn = {15292916},
year = {2019},
date = {2019-01-01},
journal = {Nature Immunology},
abstract = {Allergic skin diseases, such as atopic dermatitis, are clinically characterized by severe itching and type 2 immunity-associated hypersensitivity to widely distributed allergens, including those derived from house dust mites (HDMs). Here we found that HDMs with cysteine protease activity directly activated peptidergic nociceptors, which are neuropeptide-producing nociceptive sensory neurons that express the ion channel TRPV1 and Tac1, the gene encoding the precursor for the neuropeptide substance P. Intravital imaging and genetic approaches indicated that HDM-activated nociceptors drive the development of allergic skin inflammation by inducing the degranulation of mast cells contiguous to such nociceptors, through the release of substance P and the activation of the cationic molecule receptor MRGPRB2 on mast cells. These data indicate that, after exposure to HDM allergens, activation of TRPV1+Tac1+ nociceptor–MRGPRB2+ mast cell sensory clusters represents a key early event in the development of allergic skin reactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Allergic skin diseases, such as atopic dermatitis, are clinically characterized by severe itching and type 2 immunity-associated hypersensitivity to widely distributed allergens, including those derived from house dust mites (HDMs). Here we found that HDMs with cysteine protease activity directly activated peptidergic nociceptors, which are neuropeptide-producing nociceptive sensory neurons that express the ion channel TRPV1 and Tac1, the gene encoding the precursor for the neuropeptide substance P. Intravital imaging and genetic approaches indicated that HDM-activated nociceptors drive the development of allergic skin inflammation by inducing the degranulation of mast cells contiguous to such nociceptors, through the release of substance P and the activation of the cationic molecule receptor MRGPRB2 on mast cells. These data indicate that, after exposure to HDM allergens, activation of TRPV1+Tac1+ nociceptor–MRGPRB2+ mast cell sensory clusters represents a key early event in the development of allergic skin reactions. |
Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L. L.; Marichal, T.; Starkl, P.; Cenac, N.; Dong, X.; Tsai, M.; Galli, S. J.; Gaudenzio, N. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation Article de journal Dans: Nat Immunol, vol. 20, no. 11, p. 1435-1443, 2019, (Serhan, Nadine
Basso, Lilian
Sibilano, Riccardo
Petitfils, Camille
Meixiong, James
Bonnart, Chrystelle
Reber, Laurent L
Marichal, Thomas
Starkl, Philipp
Cenac, Nicolas
Dong, Xinzhong
Tsai, Mindy
Galli, Stephen J
Gaudenzio, Nicolas
U19 AI104209/AI/NIAID NIH HHS/United States
P30 NS069375/NS/NINDS NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
802041/ERC_/European Research Council/International
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Immunol. 2019 Nov;20(11):1435-1443. doi: 10.1038/s41590-019-0493-z. Epub 2019 Oct 7.). @article{d,
title = {House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation},
author = {Serhan, N. and Basso, L. and Sibilano, R. and Petitfils, C. and Meixiong, J. and Bonnart, C. and Reber, L. L. and Marichal, T. and Starkl, P. and Cenac, N. and Dong, X. and Tsai, M. and Galli, S. J. and Gaudenzio, N.},
year = {2019},
date = {2019-01-01},
journal = {Nat Immunol},
volume = {20},
number = {11},
pages = {1435-1443},
abstract = {Allergic skin diseases, such as atopic dermatitis, are clinically characterized by severe itching and type 2 immunity-associated hypersensitivity to widely distributed allergens, including those derived from house dust mites (HDMs). Here we found that HDMs with cysteine protease activity directly activated peptidergic nociceptors, which are neuropeptide-producing nociceptive sensory neurons that express the ion channel TRPV1 and Tac1, the gene encoding the precursor for the neuropeptide substance P. Intravital imaging and genetic approaches indicated that HDM-activated nociceptors drive the development of allergic skin inflammation by inducing the degranulation of mast cells contiguous to such nociceptors, through the release of substance P and the activation of the cationic molecule receptor MRGPRB2 on mast cells. These data indicate that, after exposure to HDM allergens, activation of TRPV1(+)Tac1(+) nociceptor-MRGPRB2(+) mast cell sensory clusters represents a key early event in the development of allergic skin reactions.},
note = {Serhan, Nadine
Basso, Lilian
Sibilano, Riccardo
Petitfils, Camille
Meixiong, James
Bonnart, Chrystelle
Reber, Laurent L
Marichal, Thomas
Starkl, Philipp
Cenac, Nicolas
Dong, Xinzhong
Tsai, Mindy
Galli, Stephen J
Gaudenzio, Nicolas
U19 AI104209/AI/NIAID NIH HHS/United States
P30 NS069375/NS/NINDS NIH HHS/United States
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
802041/ERC_/European Research Council/International
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Immunol. 2019 Nov;20(11):1435-1443. doi: 10.1038/s41590-019-0493-z. Epub 2019 Oct 7.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Allergic skin diseases, such as atopic dermatitis, are clinically characterized by severe itching and type 2 immunity-associated hypersensitivity to widely distributed allergens, including those derived from house dust mites (HDMs). Here we found that HDMs with cysteine protease activity directly activated peptidergic nociceptors, which are neuropeptide-producing nociceptive sensory neurons that express the ion channel TRPV1 and Tac1, the gene encoding the precursor for the neuropeptide substance P. Intravital imaging and genetic approaches indicated that HDM-activated nociceptors drive the development of allergic skin inflammation by inducing the degranulation of mast cells contiguous to such nociceptors, through the release of substance P and the activation of the cationic molecule receptor MRGPRB2 on mast cells. These data indicate that, after exposure to HDM allergens, activation of TRPV1(+)Tac1(+) nociceptor-MRGPRB2(+) mast cell sensory clusters represents a key early event in the development of allergic skin reactions. |
Basso, L.; Serhan, N.; Tauber, M.; Gaudenzio, N. Peripheral neurons: Master regulators of skin and mucosal immune response Article de journal Dans: Eur J Immunol, vol. 49, no. 11, p. 1984-1997, 2019, (Basso, Lilian
Serhan, Nadine
Tauber, Marie
Gaudenzio, Nicolas
Société Française d'Allergologie/International
ERC-2018-STG #802041/H2020 European Research Council/International
Société Française de Dermatologie et de Pathologie Sexuellement Transmissible/International
Institut National de la Santé et de la Recherche Médicale/International
Individual Fellowship H2020-MSCA-IF-2016 749629/H2020 Marie Skłodowska-Curie Actions/International
Research Support, Non-U.S. Gov't
Review
Germany
Eur J Immunol. 2019 Nov;49(11):1984-1997. doi: 10.1002/eji.201848027. Epub 2019 Aug 7.). @article{d,
title = {Peripheral neurons: Master regulators of skin and mucosal immune response},
author = {Basso, L. and Serhan, N. and Tauber, M. and Gaudenzio, N.},
year = {2019},
date = {2019-01-01},
journal = {Eur J Immunol},
volume = {49},
number = {11},
pages = {1984-1997},
abstract = {The body is innervated by a meshwork of heterogeneous peripheral neurons (including sensory neurons) which project virtually to all the organs. Peripheral neurons have been studied extensively in the context of their primary function of initiation of voluntary and involuntary movement, transmission of sensations and induction of appropriate behavioral response such as withdrawal to avoid tissue injury or scratching to remove irritating molecules. More recently, breakthrough articles have shown that, on top of their primary function of signal transmission to the spinal cord and brain, peripheral neurons (including afferent neurons) could directly sense environmental alarms and consequently regulate the development of various type of immune responses through the release of neuropeptides or growth factors. In this review, we discuss recent advances in the neural regulation of the immune response, both in physiological and pathological contexts by taking into account the type of organs (lungs, skin and gut), subtypes of peripheral neurons (sympathetic, nociceptive and intrinsic gut neurons) or immune cells and strains of pathogens studied. We also highlight future challenges in the field and potential therapeutic innovations targeting neuro-immune interactions.},
note = {Basso, Lilian
Serhan, Nadine
Tauber, Marie
Gaudenzio, Nicolas
Société Française d'Allergologie/International
ERC-2018-STG #802041/H2020 European Research Council/International
Société Française de Dermatologie et de Pathologie Sexuellement Transmissible/International
Institut National de la Santé et de la Recherche Médicale/International
Individual Fellowship H2020-MSCA-IF-2016 749629/H2020 Marie Skłodowska-Curie Actions/International
Research Support, Non-U.S. Gov't
Review
Germany
Eur J Immunol. 2019 Nov;49(11):1984-1997. doi: 10.1002/eji.201848027. Epub 2019 Aug 7.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The body is innervated by a meshwork of heterogeneous peripheral neurons (including sensory neurons) which project virtually to all the organs. Peripheral neurons have been studied extensively in the context of their primary function of initiation of voluntary and involuntary movement, transmission of sensations and induction of appropriate behavioral response such as withdrawal to avoid tissue injury or scratching to remove irritating molecules. More recently, breakthrough articles have shown that, on top of their primary function of signal transmission to the spinal cord and brain, peripheral neurons (including afferent neurons) could directly sense environmental alarms and consequently regulate the development of various type of immune responses through the release of neuropeptides or growth factors. In this review, we discuss recent advances in the neural regulation of the immune response, both in physiological and pathological contexts by taking into account the type of organs (lungs, skin and gut), subtypes of peripheral neurons (sympathetic, nociceptive and intrinsic gut neurons) or immune cells and strains of pathogens studied. We also highlight future challenges in the field and potential therapeutic innovations targeting neuro-immune interactions. |
2018
|
Galli, S. J.; Gaudenzio, N. Human mast cells as antigen-presenting cells: When is this role important in vivo? Article de journal Dans: J Allergy Clin Immunol, vol. 141, no. 1, p. 92-93, 2018, (Galli, Stephen J
Gaudenzio, Nicolas
Comment
Editorial
United States
J Allergy Clin Immunol. 2018 Jan;141(1):92-93. doi: 10.1016/j.jaci.2017.05.029. Epub 2017 Jun 15.). @article{d,
title = {Human mast cells as antigen-presenting cells: When is this role important in vivo?},
author = {Galli, S. J. and Gaudenzio, N.},
year = {2018},
date = {2018-01-01},
journal = {J Allergy Clin Immunol},
volume = {141},
number = {1},
pages = {92-93},
note = {Galli, Stephen J
Gaudenzio, Nicolas
Comment
Editorial
United States
J Allergy Clin Immunol. 2018 Jan;141(1):92-93. doi: 10.1016/j.jaci.2017.05.029. Epub 2017 Jun 15.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Gaudenzio, N.; Marichal, T.; Galli, S. J.; Reber, L. L. Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity Article de journal Dans: Front Immunol, vol. 9, p. 1275, 2018, (Gaudenzio, Nicolas
Marichal, Thomas
Galli, Stephen J
Reber, Laurent L
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Research Support, U.S. Gov't, Non-P.H.S.
Review
Front Immunol. 2018 Jun 5;9:1275. doi: 10.3389/fimmu.2018.01275. eCollection 2018.). @article{d,
title = {Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity},
author = {Gaudenzio, N. and Marichal, T. and Galli, S. J. and Reber, L. L.},
year = {2018},
date = {2018-01-01},
journal = {Front Immunol},
volume = {9},
pages = {1275},
abstract = {Contact hypersensitivity (CHS) is a common T cell-mediated skin disease induced by epicutaneous sensitization to haptens. Mast cells (MCs) are widely deployed in the skin and can be activated during CHS responses to secrete diverse products, including some with pro-inflammatory and anti-inflammatory functions. Conflicting results have been obtained regarding pathogenic versus protective roles of MCs in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. This review discusses recent advances in the development and analysis of mouse models to investigate the roles of MCs and MC-associated products in vivo. Notably, fluorescent avidin-based two-photon imaging approaches enable in vivo selective labeling and simultaneous tracking of MC secretory granules (e.g., during MC degranulation) and MC gene activation by real-time longitudinal intravital microscopy in living mice. The combination of such genetic and imaging tools has shed new light on the controversial role played by MCs in mouse models of CHS. On the one hand, they can amplify CHS responses of mild severity while, on the other hand, can limit the inflammation and tissue injury associated with more severe or chronic models, in part by representing an initial source of the anti-inflammatory cytokine IL-10.},
note = {Gaudenzio, Nicolas
Marichal, Thomas
Galli, Stephen J
Reber, Laurent L
R01 AI132494/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Research Support, U.S. Gov't, Non-P.H.S.
Review
Front Immunol. 2018 Jun 5;9:1275. doi: 10.3389/fimmu.2018.01275. eCollection 2018.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Contact hypersensitivity (CHS) is a common T cell-mediated skin disease induced by epicutaneous sensitization to haptens. Mast cells (MCs) are widely deployed in the skin and can be activated during CHS responses to secrete diverse products, including some with pro-inflammatory and anti-inflammatory functions. Conflicting results have been obtained regarding pathogenic versus protective roles of MCs in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. This review discusses recent advances in the development and analysis of mouse models to investigate the roles of MCs and MC-associated products in vivo. Notably, fluorescent avidin-based two-photon imaging approaches enable in vivo selective labeling and simultaneous tracking of MC secretory granules (e.g., during MC degranulation) and MC gene activation by real-time longitudinal intravital microscopy in living mice. The combination of such genetic and imaging tools has shed new light on the controversial role played by MCs in mouse models of CHS. On the one hand, they can amplify CHS responses of mild severity while, on the other hand, can limit the inflammation and tissue injury associated with more severe or chronic models, in part by representing an initial source of the anti-inflammatory cytokine IL-10. |
Gaudenzio, Nicolas; Marichal, Thomas; Galli, Stephen J; Reber, Laurent L Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity. Article de journal Dans: Frontiers in immunology, vol. 9, p. 1275, 2018, ISSN: 1664-3224 (Print). @article{Gaudenzio2018,
title = {Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity.},
author = {Gaudenzio, Nicolas and Marichal, Thomas and Galli, Stephen J and Reber, Laurent L},
doi = {10.3389/fimmu.2018.01275},
issn = {1664-3224 (Print)},
year = {2018},
date = {2018-01-01},
journal = {Frontiers in immunology},
volume = {9},
pages = {1275},
abstract = {Contact hypersensitivity (CHS) is a common T cell-mediated skin disease induced by epicutaneous sensitization to haptens. Mast cells (MCs) are widely deployed in the skin and can be activated during CHS responses to secrete diverse products, including some with pro-inflammatory and anti-inflammatory functions. Conflicting results have been obtained regarding pathogenic versus protective roles of MCs in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. This review discusses recent advances in the development and analysis of mouse models to investigate the roles of MCs and MC-associated products in vivo. Notably, fluorescent avidin-based two-photon imaging approaches enable in vivo selective labeling and simultaneous tracking of MC secretory granules (e.g., during MC degranulation) and MC gene activation by real-time longitudinal intravital microscopy in living mice. The combination of such genetic and imaging tools has shed new light on the controversial role played by MCs in mouse models of CHS. On the one hand, they can amplify CHS responses of mild severity while, on the other hand, can limit the inflammation and tissue injury associated with more severe or chronic models, in part by representing an initial source of the anti-inflammatory cytokine IL-10.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Contact hypersensitivity (CHS) is a common T cell-mediated skin disease induced by epicutaneous sensitization to haptens. Mast cells (MCs) are widely deployed in the skin and can be activated during CHS responses to secrete diverse products, including some with pro-inflammatory and anti-inflammatory functions. Conflicting results have been obtained regarding pathogenic versus protective roles of MCs in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. This review discusses recent advances in the development and analysis of mouse models to investigate the roles of MCs and MC-associated products in vivo. Notably, fluorescent avidin-based two-photon imaging approaches enable in vivo selective labeling and simultaneous tracking of MC secretory granules (e.g., during MC degranulation) and MC gene activation by real-time longitudinal intravital microscopy in living mice. The combination of such genetic and imaging tools has shed new light on the controversial role played by MCs in mouse models of CHS. On the one hand, they can amplify CHS responses of mild severity while, on the other hand, can limit the inflammation and tissue injury associated with more severe or chronic models, in part by representing an initial source of the anti-inflammatory cytokine IL-10. |
Galli, Stephen J; Gaudenzio, Nicolas Human mast cells as antigen-presenting cells: When is this role important in vivo? Divers 2018, ISSN: 1097-6825 (Electronic). @misc{Galli2018,
title = {Human mast cells as antigen-presenting cells: When is this role important in vivo?},
author = {Galli, Stephen J and Gaudenzio, Nicolas},
doi = {10.1016/j.jaci.2017.05.029},
issn = {1097-6825 (Electronic)},
year = {2018},
date = {2018-01-01},
booktitle = {The Journal of allergy and clinical immunology},
volume = {141},
number = {1},
pages = {92--93},
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
|
2017
|
Reber, Laurent L; Sibilano, Riccardo; Starkl, Philipp; Roers, Axel; Grimbaldeston, Michele A; Tsai, Mindy; Gaudenzio, Nicolas; Galli, Stephen J Imaging protective mast cells in living mice during severe contact hypersensitivity. Article de journal Dans: JCI insight, vol. 2, no. 9, 2017, ISSN: 2379-3708 (Electronic). @article{Reber2017,
title = {Imaging protective mast cells in living mice during severe contact hypersensitivity.},
author = {Reber, Laurent L and Sibilano, Riccardo and Starkl, Philipp and Roers, Axel and Grimbaldeston, Michele A and Tsai, Mindy and Gaudenzio, Nicolas and Galli, Stephen J},
doi = {10.1172/jci.insight.92900},
issn = {2379-3708 (Electronic)},
year = {2017},
date = {2017-05-01},
journal = {JCI insight},
volume = {2},
number = {9},
abstract = {Contact hypersensitivity (CHS) is a common skin disease induced by epicutaneous sensitization to haptens. Conflicting results have been obtained regarding pathogenic versus protective roles of mast cells (MCs) in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. Here we describe a fluorescent imaging approach that enables in vivo selective labeling and tracking of MC secretory granules by real-time intravital 2-photon microscopy in living mice, and permits the identification of such MCs as a potential source of cytokines in different disease models. We show using this method that dermal MCs release their granules progressively into the surrounding microenvironment, but also represent an initial source of the antiinflammatory cytokine IL-10, during the early phase of severe CHS reactions. Finally, using 3 different types of MC-deficient mice, as well as mice in which IL-10 is ablated specifically in MCs, we show that IL-10 production by MCs can significantly limit the inflammation and tissue pathology observed in severe CHS reactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Contact hypersensitivity (CHS) is a common skin disease induced by epicutaneous sensitization to haptens. Conflicting results have been obtained regarding pathogenic versus protective roles of mast cells (MCs) in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. Here we describe a fluorescent imaging approach that enables in vivo selective labeling and tracking of MC secretory granules by real-time intravital 2-photon microscopy in living mice, and permits the identification of such MCs as a potential source of cytokines in different disease models. We show using this method that dermal MCs release their granules progressively into the surrounding microenvironment, but also represent an initial source of the antiinflammatory cytokine IL-10, during the early phase of severe CHS reactions. Finally, using 3 different types of MC-deficient mice, as well as mice in which IL-10 is ablated specifically in MCs, we show that IL-10 production by MCs can significantly limit the inflammation and tissue pathology observed in severe CHS reactions. |
Reber, Laurent L; Gillis, Caitlin M; Starkl, Philipp; Jönsson, Friederike; Sibilano, Riccardo; Marichal, Thomas; Gaudenzio, Nicolas; Bérard, Marion; Rogalla, Stephan; Contag, Christopher H; Bruhns, Pierre; Galli, Stephen J Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. Article de journal Dans: The Journal of experimental medicine, vol. 214, no. 5, p. 1249–1258, 2017, ISSN: 1540-9538 (Electronic). @article{Reber2017b,
title = {Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide.},
author = {Reber, Laurent L and Gillis, Caitlin M and Starkl, Philipp and J{ö}nsson, Friederike and Sibilano, Riccardo and Marichal, Thomas and Gaudenzio, Nicolas and B{é}rard, Marion and Rogalla, Stephan and Contag, Christopher H and Bruhns, Pierre and Galli, Stephen J},
doi = {10.1084/jem.20161238},
issn = {1540-9538 (Electronic)},
year = {2017},
date = {2017-05-01},
journal = {The Journal of experimental medicine},
volume = {214},
number = {5},
pages = {1249--1258},
abstract = {Neutrophils have crucial antimicrobial functions but are also thought to contribute to tissue injury upon exposure to bacterial products, such as lipopolysaccharide (LPS). To study the role of neutrophils in LPS-induced endotoxemia, we developed a new mouse model, PMN(DTR) mice, in which injection of diphtheria toxin induces selective neutrophil ablation. Using this model, we found, surprisingly, that neutrophils serve to protect the host from LPS-induced lethal inflammation. This protective role was observed in conventional and germ-free animal facilities, indicating that it does not depend on a particular microbiological environment. Blockade or genetic deletion of myeloperoxidase (MPO), a key neutrophil enzyme, significantly increased mortality after LPS challenge, and adoptive transfer experiments confirmed that neutrophil-derived MPO contributes importantly to protection from endotoxemia. Our findings imply that, in addition to their well-established antimicrobial properties, neutrophils can contribute to optimal host protection by limiting the extent of endotoxin-induced inflammation in an MPO-dependent manner.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Neutrophils have crucial antimicrobial functions but are also thought to contribute to tissue injury upon exposure to bacterial products, such as lipopolysaccharide (LPS). To study the role of neutrophils in LPS-induced endotoxemia, we developed a new mouse model, PMN(DTR) mice, in which injection of diphtheria toxin induces selective neutrophil ablation. Using this model, we found, surprisingly, that neutrophils serve to protect the host from LPS-induced lethal inflammation. This protective role was observed in conventional and germ-free animal facilities, indicating that it does not depend on a particular microbiological environment. Blockade or genetic deletion of myeloperoxidase (MPO), a key neutrophil enzyme, significantly increased mortality after LPS challenge, and adoptive transfer experiments confirmed that neutrophil-derived MPO contributes importantly to protection from endotoxemia. Our findings imply that, in addition to their well-established antimicrobial properties, neutrophils can contribute to optimal host protection by limiting the extent of endotoxin-induced inflammation in an MPO-dependent manner. |
Ho, Chia Chi M; Chhabra, Akanksha; Starkl, Philipp; Schnorr, Peter-John; Wilmes, Stephan; Moraga, Ignacio; Kwon, Hye-Sook; Gaudenzio, Nicolas; Sibilano, Riccardo; Wehrman, Tom S; Gakovic, Milica; Sockolosky, Jonathan T; Tiffany, Matthew R; Ring, Aaron M; Piehler, Jacob; Weissman, Irving L; Galli, Stephen J; Shizuru, Judith A; Garcia, K Christopher Decoupling the Functional Pleiotropy of Stem Cell Factor by Tuning c-Kit Signaling. Article de journal Dans: Cell, vol. 168, no. 6, p. 1041–1052.e18, 2017, ISSN: 1097-4172 (Electronic). @article{Ho2017,
title = {Decoupling the Functional Pleiotropy of Stem Cell Factor by Tuning c-Kit Signaling.},
author = {Ho, Chia Chi M and Chhabra, Akanksha and Starkl, Philipp and Schnorr, Peter-John and Wilmes, Stephan and Moraga, Ignacio and Kwon, Hye-Sook and Gaudenzio, Nicolas and Sibilano, Riccardo and Wehrman, Tom S and Gakovic, Milica and Sockolosky, Jonathan T and Tiffany, Matthew R and Ring, Aaron M and Piehler, Jacob and Weissman, Irving L and Galli, Stephen J and Shizuru, Judith A and Garcia, K Christopher},
doi = {10.1016/j.cell.2017.02.011},
issn = {1097-4172 (Electronic)},
year = {2017},
date = {2017-03-01},
journal = {Cell},
volume = {168},
number = {6},
pages = {1041--1052.e18},
abstract = {Most secreted growth factors and cytokines are functionally pleiotropic because their receptors are expressed on diverse cell types. While important for normal mammalian physiology, pleiotropy limits the efficacy of cytokines and growth factors as therapeutics. Stem cell factor (SCF) is a growth factor that acts through the c-Kit receptor tyrosine kinase to elicit hematopoietic progenitor expansion but can be toxic when administered in vivo because it concurrently activates mast cells. We engineered a mechanism-based SCF partial agonist that impaired c-Kit dimerization, truncating downstream signaling amplitude. This SCF variant elicited biased activation of hematopoietic progenitors over mast cells in vitro and in vivo. Mouse models of SCF-mediated anaphylaxis, radioprotection, and hematopoietic expansion revealed that this SCF partial agonist retained therapeutic efficacy while exhibiting virtually no anaphylactic off-target effects. The approach of biasing cell activation by tuning signaling thresholds and outputs has applications to many dimeric receptor-ligand systems.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Most secreted growth factors and cytokines are functionally pleiotropic because their receptors are expressed on diverse cell types. While important for normal mammalian physiology, pleiotropy limits the efficacy of cytokines and growth factors as therapeutics. Stem cell factor (SCF) is a growth factor that acts through the c-Kit receptor tyrosine kinase to elicit hematopoietic progenitor expansion but can be toxic when administered in vivo because it concurrently activates mast cells. We engineered a mechanism-based SCF partial agonist that impaired c-Kit dimerization, truncating downstream signaling amplitude. This SCF variant elicited biased activation of hematopoietic progenitors over mast cells in vitro and in vivo. Mouse models of SCF-mediated anaphylaxis, radioprotection, and hematopoietic expansion revealed that this SCF partial agonist retained therapeutic efficacy while exhibiting virtually no anaphylactic off-target effects. The approach of biasing cell activation by tuning signaling thresholds and outputs has applications to many dimeric receptor-ligand systems. |
Mukai, Kaori; Gaudenzio, Nicolas; Gupta, Sheena; Vivanco, Nora; Bendall, Sean C; Maecker, Holden T; Chinthrajah, Rebecca S; Tsai, Mindy; Nadeau, Kari C; Galli, Stephen J Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. Article de journal Dans: The Journal of allergy and clinical immunology, vol. 139, no. 3, p. 889–899.e11, 2017, ISSN: 1097-6825 (Electronic). @article{Mukai2017,
title = {Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis.},
author = {Mukai, Kaori and Gaudenzio, Nicolas and Gupta, Sheena and Vivanco, Nora and Bendall, Sean C and Maecker, Holden T and Chinthrajah, Rebecca S and Tsai, Mindy and Nadeau, Kari C and Galli, Stephen J},
doi = {10.1016/j.jaci.2016.04.060},
issn = {1097-6825 (Electronic)},
year = {2017},
date = {2017-03-01},
journal = {The Journal of allergy and clinical immunology},
volume = {139},
number = {3},
pages = {889--899.e11},
abstract = {BACKGROUND: Basophil activation tests (BATs) have promise for research and for clinical monitoring of patients with allergies. However, BAT protocols vary in blood anticoagulant used and temperature and time of storage before testing, complicating comparisons of results from various studies. OBJECTIVE: We attempted to establish a BAT protocol that would permit analysis of blood within 24 hours of obtaining the sample. METHODS: Blood from 46 healthy donors and 120 patients with peanut allergy was collected into EDTA or heparin tubes, and samples were stored at 4°C or room temperature for 4 or 24 hours before performing BATs. RESULTS: Stimulation with anti-IgE or IL-3 resulted in strong upregulation of basophil CD203c in samples collected in EDTA or heparin, stored at 4°C, and analyzed 24 hours after sample collection. However, a CD63(hi) population of basophils was not observed in any conditions in EDTA-treated samples unless exogenous calcium/magnesium was added at the time of anti-IgE stimulation. By contrast, blood samples collected in heparin tubes were adequate for quantification of upregulation of basophil CD203c and identification of a population of CD63(hi) basophils, irrespective of whether the specimens were analyzed by means of conventional flow cytometry or cytometry by time-of-flight mass spectrometry, and such tests could be performed after blood was stored for 24 hours at 4°C. CONCLUSION: BATs to measure upregulation of basophil CD203c and induction of a CD63(hi) basophil population can be conducted with blood obtained in heparin tubes and stored at 4°C for 24 hours.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Basophil activation tests (BATs) have promise for research and for clinical monitoring of patients with allergies. However, BAT protocols vary in blood anticoagulant used and temperature and time of storage before testing, complicating comparisons of results from various studies. OBJECTIVE: We attempted to establish a BAT protocol that would permit analysis of blood within 24 hours of obtaining the sample. METHODS: Blood from 46 healthy donors and 120 patients with peanut allergy was collected into EDTA or heparin tubes, and samples were stored at 4°C or room temperature for 4 or 24 hours before performing BATs. RESULTS: Stimulation with anti-IgE or IL-3 resulted in strong upregulation of basophil CD203c in samples collected in EDTA or heparin, stored at 4°C, and analyzed 24 hours after sample collection. However, a CD63(hi) population of basophils was not observed in any conditions in EDTA-treated samples unless exogenous calcium/magnesium was added at the time of anti-IgE stimulation. By contrast, blood samples collected in heparin tubes were adequate for quantification of upregulation of basophil CD203c and identification of a population of CD63(hi) basophils, irrespective of whether the specimens were analyzed by means of conventional flow cytometry or cytometry by time-of-flight mass spectrometry, and such tests could be performed after blood was stored for 24 hours at 4°C. CONCLUSION: BATs to measure upregulation of basophil CD203c and induction of a CD63(hi) basophil population can be conducted with blood obtained in heparin tubes and stored at 4°C for 24 hours. |
Balbino, Bianca; Sibilano, Riccardo; Starkl, Philipp; Marichal, Thomas; Gaudenzio, Nicolas; Karasuyama, Hajime; Bruhns, Pierre; Tsai, Mindy; Reber, Laurent L; Galli, Stephen J Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis. Article de journal Dans: The Journal of allergy and clinical immunology, vol. 139, no. 2, p. 584–596.e10, 2017, ISSN: 1097-6825 (Electronic). @article{Balbino2017,
title = {Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis.},
author = {Balbino, Bianca and Sibilano, Riccardo and Starkl, Philipp and Marichal, Thomas and Gaudenzio, Nicolas and Karasuyama, Hajime and Bruhns, Pierre and Tsai, Mindy and Reber, Laurent L and Galli, Stephen J},
doi = {10.1016/j.jaci.2016.05.047},
issn = {1097-6825 (Electronic)},
year = {2017},
date = {2017-02-01},
journal = {The Journal of allergy and clinical immunology},
volume = {139},
number = {2},
pages = {584--596.e10},
abstract = {BACKGROUND: Conflicting results have been obtained regarding the roles of Fc receptors and effector cells in models of active systemic anaphylaxis (ASA). In part, this might reflect the choice of adjuvant used during sensitization because various adjuvants might differentially influence the production of particular antibody isotypes. OBJECTIVE: We developed an "adjuvant-free" mouse model of ASA and assessed the contributions of components of the "classical" and "alternative" pathways in this model. METHODS: Mice were sensitized intraperitoneally with ovalbumin at weekly intervals for 6 weeks and challenged intraperitoneally with ovalbumin 2 weeks later. RESULTS: Wild-type animals had immediate hypothermia and late-phase intraperitoneal inflammation in this model. These features were reduced in mice lacking the IgE receptor Fc$epsilon$RI, the IgG receptor Fc$gamma$RIII or the common $gamma$-chain FcR$gamma$. Fc$gamma$RIV blockade resulted in a partial reduction of inflammation without any effect on hypothermia. Depletion of monocytes/macrophages with clodronate liposomes significantly reduced the hypothermia response. By contrast, depletion of neutrophils or basophils had no significant effects in this ASA model. Both the hypothermia and inflammation were dependent on platelet-activating factor and histamine and were reduced in 2 types of mast cell (MC)-deficient mice. Finally, engraftment of MC-deficient mice with bone marrow-derived cultured MCs significantly exacerbated the hypothermia response and restored inflammation to levels similar to those observed in wild-type mice. CONCLUSION: Components of the classical and alternative pathways contribute to anaphylaxis in this adjuvant-free model, with key roles for MCs and monocytes/macrophages.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Conflicting results have been obtained regarding the roles of Fc receptors and effector cells in models of active systemic anaphylaxis (ASA). In part, this might reflect the choice of adjuvant used during sensitization because various adjuvants might differentially influence the production of particular antibody isotypes. OBJECTIVE: We developed an "adjuvant-free" mouse model of ASA and assessed the contributions of components of the "classical" and "alternative" pathways in this model. METHODS: Mice were sensitized intraperitoneally with ovalbumin at weekly intervals for 6 weeks and challenged intraperitoneally with ovalbumin 2 weeks later. RESULTS: Wild-type animals had immediate hypothermia and late-phase intraperitoneal inflammation in this model. These features were reduced in mice lacking the IgE receptor Fc$epsilon$RI, the IgG receptor Fc$gamma$RIII or the common $gamma$-chain FcR$gamma$. Fc$gamma$RIV blockade resulted in a partial reduction of inflammation without any effect on hypothermia. Depletion of monocytes/macrophages with clodronate liposomes significantly reduced the hypothermia response. By contrast, depletion of neutrophils or basophils had no significant effects in this ASA model. Both the hypothermia and inflammation were dependent on platelet-activating factor and histamine and were reduced in 2 types of mast cell (MC)-deficient mice. Finally, engraftment of MC-deficient mice with bone marrow-derived cultured MCs significantly exacerbated the hypothermia response and restored inflammation to levels similar to those observed in wild-type mice. CONCLUSION: Components of the classical and alternative pathways contribute to anaphylaxis in this adjuvant-free model, with key roles for MCs and monocytes/macrophages. |
Mukai, K.; Gaudenzio, N.; Gupta, S.; Vivanco, N.; Bendall, S. C.; Maecker, H. T.; Chinthrajah, R. S.; Tsai, M.; Nadeau, K. C.; Galli, S. J. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis Article de journal Dans: J Allergy Clin Immunol, vol. 139, no. 3, p. 889-899 e11, 2017, (Mukai, Kaori
Gaudenzio, Nicolas
Gupta, Sheena
Vivanco, Nora
Bendall, Sean C
Maecker, Holden T
Chinthrajah, Rebecca S
Tsai, Mindy
Nadeau, Kari C
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
J Allergy Clin Immunol. 2017 Mar;139(3):889-899.e11. doi: 10.1016/j.jaci.2016.04.060. Epub 2016 Jul 15.). @article{d,
title = {Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis},
author = {Mukai, K. and Gaudenzio, N. and Gupta, S. and Vivanco, N. and Bendall, S. C. and Maecker, H. T. and Chinthrajah, R. S. and Tsai, M. and Nadeau, K. C. and Galli, S. J.},
year = {2017},
date = {2017-01-01},
journal = {J Allergy Clin Immunol},
volume = {139},
number = {3},
pages = {889-899 e11},
abstract = {BACKGROUND: Basophil activation tests (BATs) have promise for research and for clinical monitoring of patients with allergies. However, BAT protocols vary in blood anticoagulant used and temperature and time of storage before testing, complicating comparisons of results from various studies. OBJECTIVE: We attempted to establish a BAT protocol that would permit analysis of blood within 24 hours of obtaining the sample. METHODS: Blood from 46 healthy donors and 120 patients with peanut allergy was collected into EDTA or heparin tubes, and samples were stored at 4°C or room temperature for 4 or 24 hours before performing BATs. RESULTS: Stimulation with anti-IgE or IL-3 resulted in strong upregulation of basophil CD203c in samples collected in EDTA or heparin, stored at 4°C, and analyzed 24 hours after sample collection. However, a CD63(hi) population of basophils was not observed in any conditions in EDTA-treated samples unless exogenous calcium/magnesium was added at the time of anti-IgE stimulation. By contrast, blood samples collected in heparin tubes were adequate for quantification of upregulation of basophil CD203c and identification of a population of CD63(hi) basophils, irrespective of whether the specimens were analyzed by means of conventional flow cytometry or cytometry by time-of-flight mass spectrometry, and such tests could be performed after blood was stored for 24 hours at 4°C. CONCLUSION: BATs to measure upregulation of basophil CD203c and induction of a CD63(hi) basophil population can be conducted with blood obtained in heparin tubes and stored at 4°C for 24 hours.},
note = {Mukai, Kaori
Gaudenzio, Nicolas
Gupta, Sheena
Vivanco, Nora
Bendall, Sean C
Maecker, Holden T
Chinthrajah, Rebecca S
Tsai, Mindy
Nadeau, Kari C
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
J Allergy Clin Immunol. 2017 Mar;139(3):889-899.e11. doi: 10.1016/j.jaci.2016.04.060. Epub 2016 Jul 15.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Basophil activation tests (BATs) have promise for research and for clinical monitoring of patients with allergies. However, BAT protocols vary in blood anticoagulant used and temperature and time of storage before testing, complicating comparisons of results from various studies. OBJECTIVE: We attempted to establish a BAT protocol that would permit analysis of blood within 24 hours of obtaining the sample. METHODS: Blood from 46 healthy donors and 120 patients with peanut allergy was collected into EDTA or heparin tubes, and samples were stored at 4°C or room temperature for 4 or 24 hours before performing BATs. RESULTS: Stimulation with anti-IgE or IL-3 resulted in strong upregulation of basophil CD203c in samples collected in EDTA or heparin, stored at 4°C, and analyzed 24 hours after sample collection. However, a CD63(hi) population of basophils was not observed in any conditions in EDTA-treated samples unless exogenous calcium/magnesium was added at the time of anti-IgE stimulation. By contrast, blood samples collected in heparin tubes were adequate for quantification of upregulation of basophil CD203c and identification of a population of CD63(hi) basophils, irrespective of whether the specimens were analyzed by means of conventional flow cytometry or cytometry by time-of-flight mass spectrometry, and such tests could be performed after blood was stored for 24 hours at 4°C. CONCLUSION: BATs to measure upregulation of basophil CD203c and induction of a CD63(hi) basophil population can be conducted with blood obtained in heparin tubes and stored at 4°C for 24 hours. |
Ho, C. C. M.; Chhabra, A.; Starkl, P.; Schnorr, P. J.; Wilmes, S.; Moraga, I.; Kwon, H. S.; Gaudenzio, N.; Sibilano, R.; Wehrman, T. S.; Gakovic, M.; Sockolosky, J. T.; Tiffany, M. R.; Ring, A. M.; Piehler, J.; Weissman, I. L.; Galli, S. J.; Shizuru, J. A.; Garcia, K. C. Decoupling the Functional Pleiotropy of Stem Cell Factor by Tuning c-Kit Signaling Article de journal Dans: Cell, vol. 168, no. 6, p. 1041-1052 e18, 2017, (Ho, Chia Chi M
Chhabra, Akanksha
Starkl, Philipp
Schnorr, Peter-John
Wilmes, Stephan
Moraga, Ignacio
Kwon, Hye-Sook
Gaudenzio, Nicolas
Sibilano, Riccardo
Wehrman, Tom S
Gakovic, Milica
Sockolosky, Jonathan T
Tiffany, Matthew R
Ring, Aaron M
Piehler, Jacob
Weissman, Irving L
Galli, Stephen J
Shizuru, Judith A
Garcia, K Christopher
R01 CA177684/CA/NCI NIH HHS/United States
R37 AI051321/AI/NIAID NIH HHS/United States
R43 AI072905/AI/NIAID NIH HHS/United States
HHMI/Howard Hughes Medical Institute/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Cell. 2017 Mar 9;168(6):1041-1052.e18. doi: 10.1016/j.cell.2017.02.011.). @article{d,
title = {Decoupling the Functional Pleiotropy of Stem Cell Factor by Tuning c-Kit Signaling},
author = {Ho, C. C. M. and Chhabra, A. and Starkl, P. and Schnorr, P. J. and Wilmes, S. and Moraga, I. and Kwon, H. S. and Gaudenzio, N. and Sibilano, R. and Wehrman, T. S. and Gakovic, M. and Sockolosky, J. T. and Tiffany, M. R. and Ring, A. M. and Piehler, J. and Weissman, I. L. and Galli, S. J. and Shizuru, J. A. and Garcia, K. C.},
year = {2017},
date = {2017-01-01},
journal = {Cell},
volume = {168},
number = {6},
pages = {1041-1052 e18},
abstract = {Most secreted growth factors and cytokines are functionally pleiotropic because their receptors are expressed on diverse cell types. While important for normal mammalian physiology, pleiotropy limits the efficacy of cytokines and growth factors as therapeutics. Stem cell factor (SCF) is a growth factor that acts through the c-Kit receptor tyrosine kinase to elicit hematopoietic progenitor expansion but can be toxic when administered in vivo because it concurrently activates mast cells. We engineered a mechanism-based SCF partial agonist that impaired c-Kit dimerization, truncating downstream signaling amplitude. This SCF variant elicited biased activation of hematopoietic progenitors over mast cells in vitro and in vivo. Mouse models of SCF-mediated anaphylaxis, radioprotection, and hematopoietic expansion revealed that this SCF partial agonist retained therapeutic efficacy while exhibiting virtually no anaphylactic off-target effects. The approach of biasing cell activation by tuning signaling thresholds and outputs has applications to many dimeric receptor-ligand systems.},
note = {Ho, Chia Chi M
Chhabra, Akanksha
Starkl, Philipp
Schnorr, Peter-John
Wilmes, Stephan
Moraga, Ignacio
Kwon, Hye-Sook
Gaudenzio, Nicolas
Sibilano, Riccardo
Wehrman, Tom S
Gakovic, Milica
Sockolosky, Jonathan T
Tiffany, Matthew R
Ring, Aaron M
Piehler, Jacob
Weissman, Irving L
Galli, Stephen J
Shizuru, Judith A
Garcia, K Christopher
R01 CA177684/CA/NCI NIH HHS/United States
R37 AI051321/AI/NIAID NIH HHS/United States
R43 AI072905/AI/NIAID NIH HHS/United States
HHMI/Howard Hughes Medical Institute/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Cell. 2017 Mar 9;168(6):1041-1052.e18. doi: 10.1016/j.cell.2017.02.011.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Most secreted growth factors and cytokines are functionally pleiotropic because their receptors are expressed on diverse cell types. While important for normal mammalian physiology, pleiotropy limits the efficacy of cytokines and growth factors as therapeutics. Stem cell factor (SCF) is a growth factor that acts through the c-Kit receptor tyrosine kinase to elicit hematopoietic progenitor expansion but can be toxic when administered in vivo because it concurrently activates mast cells. We engineered a mechanism-based SCF partial agonist that impaired c-Kit dimerization, truncating downstream signaling amplitude. This SCF variant elicited biased activation of hematopoietic progenitors over mast cells in vitro and in vivo. Mouse models of SCF-mediated anaphylaxis, radioprotection, and hematopoietic expansion revealed that this SCF partial agonist retained therapeutic efficacy while exhibiting virtually no anaphylactic off-target effects. The approach of biasing cell activation by tuning signaling thresholds and outputs has applications to many dimeric receptor-ligand systems. |
Balbino, B.; Sibilano, R.; Starkl, P.; Marichal, T.; Gaudenzio, N.; Karasuyama, H.; Bruhns, P.; Tsai, M.; Reber, L. L.; Galli, S. J. Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis Article de journal Dans: J Allergy Clin Immunol, vol. 139, no. 2, p. 584-596 e10, 2017, (Balbino, Bianca
Sibilano, Riccardo
Starkl, Philipp
Marichal, Thomas
Gaudenzio, Nicolas
Karasuyama, Hajime
Bruhns, Pierre
Tsai, Mindy
Reber, Laurent L
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Allergy Clin Immunol. 2017 Feb;139(2):584-596.e10. doi: 10.1016/j.jaci.2016.05.047. Epub 2016 Jul 17.). @article{d,
title = {Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis},
author = {Balbino, B. and Sibilano, R. and Starkl, P. and Marichal, T. and Gaudenzio, N. and Karasuyama, H. and Bruhns, P. and Tsai, M. and Reber, L. L. and Galli, S. J.},
year = {2017},
date = {2017-01-01},
journal = {J Allergy Clin Immunol},
volume = {139},
number = {2},
pages = {584-596 e10},
abstract = {BACKGROUND: Conflicting results have been obtained regarding the roles of Fc receptors and effector cells in models of active systemic anaphylaxis (ASA). In part, this might reflect the choice of adjuvant used during sensitization because various adjuvants might differentially influence the production of particular antibody isotypes. OBJECTIVE: We developed an "adjuvant-free" mouse model of ASA and assessed the contributions of components of the "classical" and "alternative" pathways in this model. METHODS: Mice were sensitized intraperitoneally with ovalbumin at weekly intervals for 6 weeks and challenged intraperitoneally with ovalbumin 2 weeks later. RESULTS: Wild-type animals had immediate hypothermia and late-phase intraperitoneal inflammation in this model. These features were reduced in mice lacking the IgE receptor FcεRI, the IgG receptor FcγRIII or the common γ-chain FcRγ. FcγRIV blockade resulted in a partial reduction of inflammation without any effect on hypothermia. Depletion of monocytes/macrophages with clodronate liposomes significantly reduced the hypothermia response. By contrast, depletion of neutrophils or basophils had no significant effects in this ASA model. Both the hypothermia and inflammation were dependent on platelet-activating factor and histamine and were reduced in 2 types of mast cell (MC)-deficient mice. Finally, engraftment of MC-deficient mice with bone marrow-derived cultured MCs significantly exacerbated the hypothermia response and restored inflammation to levels similar to those observed in wild-type mice. CONCLUSION: Components of the classical and alternative pathways contribute to anaphylaxis in this adjuvant-free model, with key roles for MCs and monocytes/macrophages.},
note = {Balbino, Bianca
Sibilano, Riccardo
Starkl, Philipp
Marichal, Thomas
Gaudenzio, Nicolas
Karasuyama, Hajime
Bruhns, Pierre
Tsai, Mindy
Reber, Laurent L
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Allergy Clin Immunol. 2017 Feb;139(2):584-596.e10. doi: 10.1016/j.jaci.2016.05.047. Epub 2016 Jul 17.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Conflicting results have been obtained regarding the roles of Fc receptors and effector cells in models of active systemic anaphylaxis (ASA). In part, this might reflect the choice of adjuvant used during sensitization because various adjuvants might differentially influence the production of particular antibody isotypes. OBJECTIVE: We developed an "adjuvant-free" mouse model of ASA and assessed the contributions of components of the "classical" and "alternative" pathways in this model. METHODS: Mice were sensitized intraperitoneally with ovalbumin at weekly intervals for 6 weeks and challenged intraperitoneally with ovalbumin 2 weeks later. RESULTS: Wild-type animals had immediate hypothermia and late-phase intraperitoneal inflammation in this model. These features were reduced in mice lacking the IgE receptor FcεRI, the IgG receptor FcγRIII or the common γ-chain FcRγ. FcγRIV blockade resulted in a partial reduction of inflammation without any effect on hypothermia. Depletion of monocytes/macrophages with clodronate liposomes significantly reduced the hypothermia response. By contrast, depletion of neutrophils or basophils had no significant effects in this ASA model. Both the hypothermia and inflammation were dependent on platelet-activating factor and histamine and were reduced in 2 types of mast cell (MC)-deficient mice. Finally, engraftment of MC-deficient mice with bone marrow-derived cultured MCs significantly exacerbated the hypothermia response and restored inflammation to levels similar to those observed in wild-type mice. CONCLUSION: Components of the classical and alternative pathways contribute to anaphylaxis in this adjuvant-free model, with key roles for MCs and monocytes/macrophages. |
Reber, L. L.; Sibilano, R.; Starkl, P.; Roers, A.; Grimbaldeston, M. A.; Tsai, M.; Gaudenzio, N.; Galli, S. J. Imaging protective mast cells in living mice during severe contact hypersensitivity Article de journal Dans: JCI Insight, vol. 2, no. 9, 2017, (Reber, Laurent L
Sibilano, Riccardo
Starkl, Philipp
Roers, Axel
Grimbaldeston, Michele A
Tsai, Mindy
Gaudenzio, Nicolas
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
JCI Insight. 2017 May 4;2(9):e92900. doi: 10.1172/jci.insight.92900. eCollection 2017 May 4.). @article{d,
title = {Imaging protective mast cells in living mice during severe contact hypersensitivity},
author = {Reber, L. L. and Sibilano, R. and Starkl, P. and Roers, A. and Grimbaldeston, M. A. and Tsai, M. and Gaudenzio, N. and Galli, S. J.},
year = {2017},
date = {2017-01-01},
journal = {JCI Insight},
volume = {2},
number = {9},
abstract = {Contact hypersensitivity (CHS) is a common skin disease induced by epicutaneous sensitization to haptens. Conflicting results have been obtained regarding pathogenic versus protective roles of mast cells (MCs) in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. Here we describe a fluorescent imaging approach that enables in vivo selective labeling and tracking of MC secretory granules by real-time intravital 2-photon microscopy in living mice, and permits the identification of such MCs as a potential source of cytokines in different disease models. We show using this method that dermal MCs release their granules progressively into the surrounding microenvironment, but also represent an initial source of the antiinflammatory cytokine IL-10, during the early phase of severe CHS reactions. Finally, using 3 different types of MC-deficient mice, as well as mice in which IL-10 is ablated specifically in MCs, we show that IL-10 production by MCs can significantly limit the inflammation and tissue pathology observed in severe CHS reactions.},
note = {Reber, Laurent L
Sibilano, Riccardo
Starkl, Philipp
Roers, Axel
Grimbaldeston, Michele A
Tsai, Mindy
Gaudenzio, Nicolas
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
JCI Insight. 2017 May 4;2(9):e92900. doi: 10.1172/jci.insight.92900. eCollection 2017 May 4.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Contact hypersensitivity (CHS) is a common skin disease induced by epicutaneous sensitization to haptens. Conflicting results have been obtained regarding pathogenic versus protective roles of mast cells (MCs) in CHS, and this has been attributed in part to the limitations of certain models for studying MC functions in vivo. Here we describe a fluorescent imaging approach that enables in vivo selective labeling and tracking of MC secretory granules by real-time intravital 2-photon microscopy in living mice, and permits the identification of such MCs as a potential source of cytokines in different disease models. We show using this method that dermal MCs release their granules progressively into the surrounding microenvironment, but also represent an initial source of the antiinflammatory cytokine IL-10, during the early phase of severe CHS reactions. Finally, using 3 different types of MC-deficient mice, as well as mice in which IL-10 is ablated specifically in MCs, we show that IL-10 production by MCs can significantly limit the inflammation and tissue pathology observed in severe CHS reactions. |
Reber, L. L.; Gillis, C. M.; Starkl, P.; Jönsson, F.; Sibilano, R.; Marichal, T.; Gaudenzio, N.; Bérard, M.; Rogalla, S.; Contag, C. H.; Bruhns, P.; Galli, S. J. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide Article de journal Dans: J Exp Med, vol. 214, no. 5, p. 1249-1258, 2017, (Reber, Laurent L
Gillis, Caitlin M
Starkl, Philipp
Jönsson, Friederike
Sibilano, Riccardo
Marichal, Thomas
Gaudenzio, Nicolas
Bérard, Marion
Rogalla, Stephan
Contag, Christopher H
Bruhns, Pierre
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R21 NS080062/NS/NINDS NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Exp Med. 2017 May 1;214(5):1249-1258. doi: 10.1084/jem.20161238. Epub 2017 Apr 6.). @article{d,
title = {Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide},
author = {Reber, L. L. and Gillis, C. M. and Starkl, P. and Jönsson, F. and Sibilano, R. and Marichal, T. and Gaudenzio, N. and Bérard, M. and Rogalla, S. and Contag, C. H. and Bruhns, P. and Galli, S. J.},
year = {2017},
date = {2017-01-01},
journal = {J Exp Med},
volume = {214},
number = {5},
pages = {1249-1258},
abstract = {Neutrophils have crucial antimicrobial functions but are also thought to contribute to tissue injury upon exposure to bacterial products, such as lipopolysaccharide (LPS). To study the role of neutrophils in LPS-induced endotoxemia, we developed a new mouse model, PMN(DTR) mice, in which injection of diphtheria toxin induces selective neutrophil ablation. Using this model, we found, surprisingly, that neutrophils serve to protect the host from LPS-induced lethal inflammation. This protective role was observed in conventional and germ-free animal facilities, indicating that it does not depend on a particular microbiological environment. Blockade or genetic deletion of myeloperoxidase (MPO), a key neutrophil enzyme, significantly increased mortality after LPS challenge, and adoptive transfer experiments confirmed that neutrophil-derived MPO contributes importantly to protection from endotoxemia. Our findings imply that, in addition to their well-established antimicrobial properties, neutrophils can contribute to optimal host protection by limiting the extent of endotoxin-induced inflammation in an MPO-dependent manner.},
note = {Reber, Laurent L
Gillis, Caitlin M
Starkl, Philipp
Jönsson, Friederike
Sibilano, Riccardo
Marichal, Thomas
Gaudenzio, Nicolas
Bérard, Marion
Rogalla, Stephan
Contag, Christopher H
Bruhns, Pierre
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R21 NS080062/NS/NINDS NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Exp Med. 2017 May 1;214(5):1249-1258. doi: 10.1084/jem.20161238. Epub 2017 Apr 6.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Neutrophils have crucial antimicrobial functions but are also thought to contribute to tissue injury upon exposure to bacterial products, such as lipopolysaccharide (LPS). To study the role of neutrophils in LPS-induced endotoxemia, we developed a new mouse model, PMN(DTR) mice, in which injection of diphtheria toxin induces selective neutrophil ablation. Using this model, we found, surprisingly, that neutrophils serve to protect the host from LPS-induced lethal inflammation. This protective role was observed in conventional and germ-free animal facilities, indicating that it does not depend on a particular microbiological environment. Blockade or genetic deletion of myeloperoxidase (MPO), a key neutrophil enzyme, significantly increased mortality after LPS challenge, and adoptive transfer experiments confirmed that neutrophil-derived MPO contributes importantly to protection from endotoxemia. Our findings imply that, in addition to their well-established antimicrobial properties, neutrophils can contribute to optimal host protection by limiting the extent of endotoxin-induced inflammation in an MPO-dependent manner. |
2016
|
Sibilano, Riccardo; Gaudenzio, Nicolas; DeGorter, Marianne K; Reber, Laurent L; Hernandez, Joseph D; Starkl, Philipp M; Zurek, Oliwia W; Tsai, Mindy; Zahner, Sonja; Montgomery, Stephen B; Roers, Axel; Kronenberg, Mitchell; Yu, Mang; Galli, Stephen J A TNFRSF14-FcɛRI-mast cell pathway contributes to development of multiple features of asthma pathology in mice. Article de journal Dans: Nature communications, vol. 7, p. 13696, 2016, ISSN: 2041-1723 (Electronic). @article{Sibilano2016,
title = {A TNFRSF14-FcɛRI-mast cell pathway contributes to development of multiple features of asthma pathology in mice.},
author = {Sibilano, Riccardo and Gaudenzio, Nicolas and DeGorter, Marianne K and Reber, Laurent L and Hernandez, Joseph D and Starkl, Philipp M and Zurek, Oliwia W and Tsai, Mindy and Zahner, Sonja and Montgomery, Stephen B and Roers, Axel and Kronenberg, Mitchell and Yu, Mang and Galli, Stephen J},
doi = {10.1038/ncomms13696},
issn = {2041-1723 (Electronic)},
year = {2016},
date = {2016-12-01},
journal = {Nature communications},
volume = {7},
pages = {13696},
abstract = {Asthma has multiple features, including airway hyperreactivity, inflammation and remodelling. The TNF superfamily member TNFSF14 (LIGHT), via interactions with the receptor TNFRSF14 (HVEM), can support T(H)2 cell generation and longevity and promote airway remodelling in mouse models of asthma, but the mechanisms by which TNFSF14 functions in this setting are incompletely understood. Here we find that mouse and human mast cells (MCs) express TNFRSF14 and that TNFSF14:TNFRSF14 interactions can enhance IgE-mediated MC signalling and mediator production. In mouse models of asthma, TNFRSF14 blockade with a neutralizing antibody administered after antigen sensitization, or genetic deletion of Tnfrsf14, diminishes plasma levels of antigen-specific IgG(1) and IgE antibodies, airway hyperreactivity, airway inflammation and airway remodelling. Finally, by analysing two types of genetically MC-deficient mice after engrafting MCs that either do or do not express TNFRSF14, we show that TNFRSF14 expression on MCs significantly contributes to the development of multiple features of asthma pathology.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Asthma has multiple features, including airway hyperreactivity, inflammation and remodelling. The TNF superfamily member TNFSF14 (LIGHT), via interactions with the receptor TNFRSF14 (HVEM), can support T(H)2 cell generation and longevity and promote airway remodelling in mouse models of asthma, but the mechanisms by which TNFSF14 functions in this setting are incompletely understood. Here we find that mouse and human mast cells (MCs) express TNFRSF14 and that TNFSF14:TNFRSF14 interactions can enhance IgE-mediated MC signalling and mediator production. In mouse models of asthma, TNFRSF14 blockade with a neutralizing antibody administered after antigen sensitization, or genetic deletion of Tnfrsf14, diminishes plasma levels of antigen-specific IgG(1) and IgE antibodies, airway hyperreactivity, airway inflammation and airway remodelling. Finally, by analysing two types of genetically MC-deficient mice after engrafting MCs that either do or do not express TNFRSF14, we show that TNFRSF14 expression on MCs significantly contributes to the development of multiple features of asthma pathology. |
Marichal, Thomas; Gaudenzio, Nicolas; El Abbas, Sophie; Sibilano, Riccardo; Zurek, Oliwia; Starkl, Philipp; Reber, Laurent L; Pirottin, Dimitri; Kim, Jinah; Chambon, Pierre; Roers, Axel; Antoine, Nadine; Kawakami, Yuko; Kawakami, Toshiaki; Bureau, Fabrice; Tam, See-Ying; Tsai, Mindy; Galli, Stephen J Guanine nucleotide exchange factor RABGEF1 regulates keratinocyte-intrinsic signaling to maintain skin homeostasis. Article de journal Dans: The Journal of clinical investigation, vol. 126, no. 12, p. 4497–4515, 2016, ISSN: 1558-8238 (Electronic). @article{Marichal2016,
title = {Guanine nucleotide exchange factor RABGEF1 regulates keratinocyte-intrinsic signaling to maintain skin homeostasis.},
author = {Marichal, Thomas and Gaudenzio, Nicolas and {El Abbas}, Sophie and Sibilano, Riccardo and Zurek, Oliwia and Starkl, Philipp and Reber, Laurent L and Pirottin, Dimitri and Kim, Jinah and Chambon, Pierre and Roers, Axel and Antoine, Nadine and Kawakami, Yuko and Kawakami, Toshiaki and Bureau, Fabrice and Tam, See-Ying and Tsai, Mindy and Galli, Stephen J},
doi = {10.1172/JCI86359},
issn = {1558-8238 (Electronic)},
year = {2016},
date = {2016-12-01},
journal = {The Journal of clinical investigation},
volume = {126},
number = {12},
pages = {4497--4515},
abstract = {Epidermal keratinocytes form a structural and immune barrier that is essential for skin homeostasis. However, the mechanisms that regulate epidermal barrier function are incompletely understood. Here we have found that keratinocyte-specific deletion of the gene encoding RAB guanine nucleotide exchange factor 1 (RABGEF1, also known as RABEX-5) severely impairs epidermal barrier function in mice and induces an allergic cutaneous and systemic phenotype. RABGEF1-deficient keratinocytes exhibited aberrant activation of the intrinsic IL-1R/MYD88/NF-$kappa$B signaling pathway and MYD88-dependent abnormalities in expression of structural proteins that contribute to skin barrier function. Moreover, ablation of MYD88 signaling in RABGEF1-deficient keratinocytes or deletion of Il1r1 restored skin homeostasis and prevented development of skin inflammation. We further demonstrated that epidermal RABGEF1 expression is reduced in skin lesions of humans diagnosed with either atopic dermatitis or allergic contact dermatitis as well as in an inducible mouse model of allergic dermatitis. Our findings reveal a key role for RABGEF1 in dampening keratinocyte-intrinsic MYD88 signaling and sustaining epidermal barrier function in mice, and suggest that dysregulation of RABGEF1 expression may contribute to epidermal barrier dysfunction in allergic skin disorders in mice and humans. Thus, RABGEF1-mediated regulation of IL-1R/MYD88 signaling might represent a potential therapeutic target.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Epidermal keratinocytes form a structural and immune barrier that is essential for skin homeostasis. However, the mechanisms that regulate epidermal barrier function are incompletely understood. Here we have found that keratinocyte-specific deletion of the gene encoding RAB guanine nucleotide exchange factor 1 (RABGEF1, also known as RABEX-5) severely impairs epidermal barrier function in mice and induces an allergic cutaneous and systemic phenotype. RABGEF1-deficient keratinocytes exhibited aberrant activation of the intrinsic IL-1R/MYD88/NF-$kappa$B signaling pathway and MYD88-dependent abnormalities in expression of structural proteins that contribute to skin barrier function. Moreover, ablation of MYD88 signaling in RABGEF1-deficient keratinocytes or deletion of Il1r1 restored skin homeostasis and prevented development of skin inflammation. We further demonstrated that epidermal RABGEF1 expression is reduced in skin lesions of humans diagnosed with either atopic dermatitis or allergic contact dermatitis as well as in an inducible mouse model of allergic dermatitis. Our findings reveal a key role for RABGEF1 in dampening keratinocyte-intrinsic MYD88 signaling and sustaining epidermal barrier function in mice, and suggest that dysregulation of RABGEF1 expression may contribute to epidermal barrier dysfunction in allergic skin disorders in mice and humans. Thus, RABGEF1-mediated regulation of IL-1R/MYD88 signaling might represent a potential therapeutic target. |
Reber, Laurent L; Gaudenzio, Nicolas; Starkl, Philipp; Galli, Stephen J Neutrophils are not required for resolution of acute gouty arthritis in mice. Divers 2016, ISSN: 1546-170X (Electronic). @misc{Reber2016,
title = {Neutrophils are not required for resolution of acute gouty arthritis in mice.},
author = {Reber, Laurent L and Gaudenzio, Nicolas and Starkl, Philipp and Galli, Stephen J},
doi = {10.1038/nm.4216},
issn = {1546-170X (Electronic)},
year = {2016},
date = {2016-12-01},
booktitle = {Nature medicine},
volume = {22},
number = {12},
pages = {1382--1384},
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
|
Gaudenzio, Nicolas; Sibilano, Riccardo; Marichal, Thomas; Starkl, Philipp; Reber, Laurent L; Cenac, Nicolas; McNeil, Benjamin D; Dong, Xinzhong; Hernandez, Joseph D; Sagi-Eisenberg, Ronit; Hammel, Ilan; Roers, Axel; Valitutti, Salvatore; Tsai, Mindy; Espinosa, Eric; Galli, Stephen J Different activation signals induce distinct mast cell degranulation strategies. Article de journal Dans: The Journal of clinical investigation, vol. 126, no. 10, p. 3981–3998, 2016, ISSN: 1558-8238 (Electronic). @article{Gaudenzio2016,
title = {Different activation signals induce distinct mast cell degranulation strategies.},
author = {Gaudenzio, Nicolas and Sibilano, Riccardo and Marichal, Thomas and Starkl, Philipp and Reber, Laurent L and Cenac, Nicolas and McNeil, Benjamin D and Dong, Xinzhong and Hernandez, Joseph D and Sagi-Eisenberg, Ronit and Hammel, Ilan and Roers, Axel and Valitutti, Salvatore and Tsai, Mindy and Espinosa, Eric and Galli, Stephen J},
doi = {10.1172/JCI85538},
issn = {1558-8238 (Electronic)},
year = {2016},
date = {2016-10-01},
journal = {The Journal of clinical investigation},
volume = {126},
number = {10},
pages = {3981--3998},
abstract = {Mast cells (MCs) influence intercellular communication during inflammation by secreting cytoplasmic granules that contain diverse mediators. Here, we have demonstrated that MCs decode different activation stimuli into spatially and temporally distinct patterns of granule secretion. Certain signals, including substance P, the complement anaphylatoxins C3a and C5a, and endothelin 1, induced human MCs rapidly to secrete small and relatively spherical granule structures, a pattern consistent with the secretion of individual granules. Conversely, activating MCs with anti-IgE increased the time partition between signaling and secretion, which was associated with a period of sustained elevation of intracellular calcium and formation of larger and more heterogeneously shaped granule structures that underwent prolonged exteriorization. Pharmacological inhibition of IKK-$beta$ during IgE-dependent stimulation strongly reduced the time partition between signaling and secretion, inhibited SNAP23/STX4 complex formation, and switched the degranulation pattern into one that resembled degranulation induced by substance P. IgE-dependent and substance P-dependent activation in vivo also induced different patterns of mouse MC degranulation that were associated with distinct local and systemic pathophysiological responses. These findings show that cytoplasmic granule secretion from MCs that occurs in response to different activating stimuli can exhibit distinct dynamics and features that are associated with distinct patterns of MC-dependent inflammation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells (MCs) influence intercellular communication during inflammation by secreting cytoplasmic granules that contain diverse mediators. Here, we have demonstrated that MCs decode different activation stimuli into spatially and temporally distinct patterns of granule secretion. Certain signals, including substance P, the complement anaphylatoxins C3a and C5a, and endothelin 1, induced human MCs rapidly to secrete small and relatively spherical granule structures, a pattern consistent with the secretion of individual granules. Conversely, activating MCs with anti-IgE increased the time partition between signaling and secretion, which was associated with a period of sustained elevation of intracellular calcium and formation of larger and more heterogeneously shaped granule structures that underwent prolonged exteriorization. Pharmacological inhibition of IKK-$beta$ during IgE-dependent stimulation strongly reduced the time partition between signaling and secretion, inhibited SNAP23/STX4 complex formation, and switched the degranulation pattern into one that resembled degranulation induced by substance P. IgE-dependent and substance P-dependent activation in vivo also induced different patterns of mouse MC degranulation that were associated with distinct local and systemic pathophysiological responses. These findings show that cytoplasmic granule secretion from MCs that occurs in response to different activating stimuli can exhibit distinct dynamics and features that are associated with distinct patterns of MC-dependent inflammation. |
Khazen, Roxana; Müller, Sabina; Gaudenzio, Nicolas; Espinosa, Eric; Puissegur, Marie-Pierre; Valitutti, Salvatore Melanoma cell lysosome secretory burst neutralizes the CTL-mediated cytotoxicity at the lytic synapse. Article de journal Dans: Nature communications, vol. 7, p. 10823, 2016, ISSN: 2041-1723 (Electronic). @article{Khazen2016,
title = {Melanoma cell lysosome secretory burst neutralizes the CTL-mediated cytotoxicity at the lytic synapse.},
author = {Khazen, Roxana and M{ü}ller, Sabina and Gaudenzio, Nicolas and Espinosa, Eric and Puissegur, Marie-Pierre and Valitutti, Salvatore},
doi = {10.1038/ncomms10823},
issn = {2041-1723 (Electronic)},
year = {2016},
date = {2016-03-01},
journal = {Nature communications},
volume = {7},
pages = {10823},
abstract = {Human melanoma cells express various tumour antigens that are recognized by CD8(+) cytotoxic T lymphocytes (CTLs) and elicit tumour-specific responses in vivo. However, natural and therapeutically enhanced CTL responses in melanoma patients are of limited efficacy. The mechanisms underlying CTL effector phase failure when facing melanomas are still largely elusive. Here we show that, on conjugation with CTL, human melanoma cells undergo an active late endosome/lysosome trafficking, which is intensified at the lytic synapse and is paralleled by cathepsin-mediated perforin degradation and deficient granzyme B penetration. Abortion of SNAP-23-dependent lysosomal trafficking, pH perturbation or impairment of lysosomal proteolytic activity restores susceptibility to CTL attack. Inside the arsenal of melanoma cell strategies to escape immune surveillance, we identify a self-defence mechanism based on exacerbated lysosome secretion and perforin degradation at the lytic synapse. Interfering with this synaptic self-defence mechanism might be useful in potentiating CTL-mediated therapies in melanoma patients.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Human melanoma cells express various tumour antigens that are recognized by CD8(+) cytotoxic T lymphocytes (CTLs) and elicit tumour-specific responses in vivo. However, natural and therapeutically enhanced CTL responses in melanoma patients are of limited efficacy. The mechanisms underlying CTL effector phase failure when facing melanomas are still largely elusive. Here we show that, on conjugation with CTL, human melanoma cells undergo an active late endosome/lysosome trafficking, which is intensified at the lytic synapse and is paralleled by cathepsin-mediated perforin degradation and deficient granzyme B penetration. Abortion of SNAP-23-dependent lysosomal trafficking, pH perturbation or impairment of lysosomal proteolytic activity restores susceptibility to CTL attack. Inside the arsenal of melanoma cell strategies to escape immune surveillance, we identify a self-defence mechanism based on exacerbated lysosome secretion and perforin degradation at the lytic synapse. Interfering with this synaptic self-defence mechanism might be useful in potentiating CTL-mediated therapies in melanoma patients. |
Starkl, Philipp; Marichal, Thomas; Gaudenzio, Nicolas; Reber, Laurent Lionel; Sibilano, Riccardo; Tsai, Mindy; Galli, Stephen Joseph IgE antibodies, Fc$epsilon$RI$alpha$, and IgE-mediated local anaphylaxis can limit snake venom toxicity. Article de journal Dans: The Journal of allergy and clinical immunology, vol. 137, no. 1, p. 246–257.e11, 2016, ISSN: 1097-6825 (Electronic). @article{Starkl2016,
title = {IgE antibodies, Fc$epsilon$RI$alpha$, and IgE-mediated local anaphylaxis can limit snake venom toxicity.},
author = {Starkl, Philipp and Marichal, Thomas and Gaudenzio, Nicolas and Reber, Laurent Lionel and Sibilano, Riccardo and Tsai, Mindy and Galli, Stephen Joseph},
doi = {10.1016/j.jaci.2015.08.005},
issn = {1097-6825 (Electronic)},
year = {2016},
date = {2016-01-01},
journal = {The Journal of allergy and clinical immunology},
volume = {137},
number = {1},
pages = {246--257.e11},
abstract = {BACKGROUND: Type 2 cytokine-related immune responses associated with development of antigen-specific IgE antibodies can contribute to pathology in patients with allergic diseases and to fatal anaphylaxis. However, recent findings in mice indicate that IgE also can enhance defense against honeybee venom. OBJECTIVE: We tested whether IgE antibodies, IgE-dependent effector mechanisms, and a local anaphylactic reaction to an unrelated antigen can enhance defense against Russell viper venom (RVV) and determined whether such responses can be influenced by immunization protocol or mouse strain. METHODS: We compared the resistance of RVV-immunized wild-type, IgE-deficient, and Fcer1a-deficient mice after injection of a potentially lethal dose of RVV. RESULTS: A single prior exposure to RVV enhanced the ability of wild-type mice, but not mice lacking IgE or functional Fc$epsilon$RI, to survive challenge with a potentially lethal amount of RVV. Moreover, IgE-dependent local passive cutaneous anaphylaxis in response to challenge with an antigen not naturally present in RVV significantly enhanced resistance to the venom. Finally, we observed different effects on resistance to RVV or honeybee venom in BALB/c versus C57BL/6 mice that had received a second exposure to that venom before challenge with a high dose of that venom. CONCLUSION: These observations illustrate the potential benefit of IgE-dependent effector mechanisms in acquired host defense against venoms. The extent to which type 2 immune responses against venoms can decrease pathology associated with envenomation seems to be influenced by the type of venom, the frequency of venom exposure, and the genetic background of the host.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Type 2 cytokine-related immune responses associated with development of antigen-specific IgE antibodies can contribute to pathology in patients with allergic diseases and to fatal anaphylaxis. However, recent findings in mice indicate that IgE also can enhance defense against honeybee venom. OBJECTIVE: We tested whether IgE antibodies, IgE-dependent effector mechanisms, and a local anaphylactic reaction to an unrelated antigen can enhance defense against Russell viper venom (RVV) and determined whether such responses can be influenced by immunization protocol or mouse strain. METHODS: We compared the resistance of RVV-immunized wild-type, IgE-deficient, and Fcer1a-deficient mice after injection of a potentially lethal dose of RVV. RESULTS: A single prior exposure to RVV enhanced the ability of wild-type mice, but not mice lacking IgE or functional Fc$epsilon$RI, to survive challenge with a potentially lethal amount of RVV. Moreover, IgE-dependent local passive cutaneous anaphylaxis in response to challenge with an antigen not naturally present in RVV significantly enhanced resistance to the venom. Finally, we observed different effects on resistance to RVV or honeybee venom in BALB/c versus C57BL/6 mice that had received a second exposure to that venom before challenge with a high dose of that venom. CONCLUSION: These observations illustrate the potential benefit of IgE-dependent effector mechanisms in acquired host defense against venoms. The extent to which type 2 immune responses against venoms can decrease pathology associated with envenomation seems to be influenced by the type of venom, the frequency of venom exposure, and the genetic background of the host. |
Starkl, P.; Marichal, T.; Gaudenzio, N.; Reber, L. L.; Sibilano, R.; Tsai, M.; Galli, S. J. IgE antibodies, FcεRIα, and IgE-mediated local anaphylaxis can limit snake venom toxicity Article de journal Dans: J Allergy Clin Immunol, vol. 137, no. 1, p. 246-257 e11, 2016, (Starkl, Philipp
Marichal, Thomas
Gaudenzio, Nicolas
Reber, Laurent Lionel
Sibilano, Riccardo
Tsai, Mindy
Galli, Stephen Joseph
R01 AI023990/AI/NIAID NIH HHS/United States
CA072074/CA/NCI NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
AI070813/AI/NIAID NIH HHS/United States
AI023990/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
K99AI110645/AI/NIAID NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
UL1 TR001085/TR/NCATS NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Allergy Clin Immunol. 2016 Jan;137(1):246-257.e11. doi: 10.1016/j.jaci.2015.08.005. Epub 2015 Sep 26.). @article{d,
title = {IgE antibodies, FcεRIα, and IgE-mediated local anaphylaxis can limit snake venom toxicity},
author = {Starkl, P. and Marichal, T. and Gaudenzio, N. and Reber, L. L. and Sibilano, R. and Tsai, M. and Galli, S. J.},
year = {2016},
date = {2016-01-01},
journal = {J Allergy Clin Immunol},
volume = {137},
number = {1},
pages = {246-257 e11},
abstract = {BACKGROUND: Type 2 cytokine-related immune responses associated with development of antigen-specific IgE antibodies can contribute to pathology in patients with allergic diseases and to fatal anaphylaxis. However, recent findings in mice indicate that IgE also can enhance defense against honeybee venom. OBJECTIVE: We tested whether IgE antibodies, IgE-dependent effector mechanisms, and a local anaphylactic reaction to an unrelated antigen can enhance defense against Russell viper venom (RVV) and determined whether such responses can be influenced by immunization protocol or mouse strain. METHODS: We compared the resistance of RVV-immunized wild-type, IgE-deficient, and Fcer1a-deficient mice after injection of a potentially lethal dose of RVV. RESULTS: A single prior exposure to RVV enhanced the ability of wild-type mice, but not mice lacking IgE or functional FcεRI, to survive challenge with a potentially lethal amount of RVV. Moreover, IgE-dependent local passive cutaneous anaphylaxis in response to challenge with an antigen not naturally present in RVV significantly enhanced resistance to the venom. Finally, we observed different effects on resistance to RVV or honeybee venom in BALB/c versus C57BL/6 mice that had received a second exposure to that venom before challenge with a high dose of that venom. CONCLUSION: These observations illustrate the potential benefit of IgE-dependent effector mechanisms in acquired host defense against venoms. The extent to which type 2 immune responses against venoms can decrease pathology associated with envenomation seems to be influenced by the type of venom, the frequency of venom exposure, and the genetic background of the host.},
note = {Starkl, Philipp
Marichal, Thomas
Gaudenzio, Nicolas
Reber, Laurent Lionel
Sibilano, Riccardo
Tsai, Mindy
Galli, Stephen Joseph
R01 AI023990/AI/NIAID NIH HHS/United States
CA072074/CA/NCI NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
AI070813/AI/NIAID NIH HHS/United States
AI023990/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
K99AI110645/AI/NIAID NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
UL1 TR001085/TR/NCATS NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Allergy Clin Immunol. 2016 Jan;137(1):246-257.e11. doi: 10.1016/j.jaci.2015.08.005. Epub 2015 Sep 26.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: Type 2 cytokine-related immune responses associated with development of antigen-specific IgE antibodies can contribute to pathology in patients with allergic diseases and to fatal anaphylaxis. However, recent findings in mice indicate that IgE also can enhance defense against honeybee venom. OBJECTIVE: We tested whether IgE antibodies, IgE-dependent effector mechanisms, and a local anaphylactic reaction to an unrelated antigen can enhance defense against Russell viper venom (RVV) and determined whether such responses can be influenced by immunization protocol or mouse strain. METHODS: We compared the resistance of RVV-immunized wild-type, IgE-deficient, and Fcer1a-deficient mice after injection of a potentially lethal dose of RVV. RESULTS: A single prior exposure to RVV enhanced the ability of wild-type mice, but not mice lacking IgE or functional FcεRI, to survive challenge with a potentially lethal amount of RVV. Moreover, IgE-dependent local passive cutaneous anaphylaxis in response to challenge with an antigen not naturally present in RVV significantly enhanced resistance to the venom. Finally, we observed different effects on resistance to RVV or honeybee venom in BALB/c versus C57BL/6 mice that had received a second exposure to that venom before challenge with a high dose of that venom. CONCLUSION: These observations illustrate the potential benefit of IgE-dependent effector mechanisms in acquired host defense against venoms. The extent to which type 2 immune responses against venoms can decrease pathology associated with envenomation seems to be influenced by the type of venom, the frequency of venom exposure, and the genetic background of the host. |
Sibilano, R.; Gaudenzio, N.; DeGorter, M. K.; Reber, L. L.; Hernandez, J. D.; Starkl, P. M.; Zurek, O. W.; Tsai, M.; Zahner, S.; Montgomery, S. B.; Roers, A.; Kronenberg, M.; Yu, M.; Galli, S. J. A TNFRSF14-FcɛRI-mast cell pathway contributes to development of multiple features of asthma pathology in mice Article de journal Dans: Nat Commun, vol. 7, p. 13696, 2016, (Sibilano, Riccardo
Gaudenzio, Nicolas
DeGorter, Marianne K
Reber, Laurent L
Hernandez, Joseph D
Starkl, Philipp M
Zurek, Oliwia W
Tsai, Mindy
Zahner, Sonja
Montgomery, Stephen B
Roers, Axel
Kronenberg, Mitchell
Yu, Mang
Galli, Stephen J
T32 AI007290/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Commun. 2016 Dec 16;7:13696. doi: 10.1038/ncomms13696.). @article{d,
title = {A TNFRSF14-FcɛRI-mast cell pathway contributes to development of multiple features of asthma pathology in mice},
author = {Sibilano, R. and Gaudenzio, N. and DeGorter, M. K. and Reber, L. L. and Hernandez, J. D. and Starkl, P. M. and Zurek, O. W. and Tsai, M. and Zahner, S. and Montgomery, S. B. and Roers, A. and Kronenberg, M. and Yu, M. and Galli, S. J.},
year = {2016},
date = {2016-01-01},
journal = {Nat Commun},
volume = {7},
pages = {13696},
abstract = {Asthma has multiple features, including airway hyperreactivity, inflammation and remodelling. The TNF superfamily member TNFSF14 (LIGHT), via interactions with the receptor TNFRSF14 (HVEM), can support T(H)2 cell generation and longevity and promote airway remodelling in mouse models of asthma, but the mechanisms by which TNFSF14 functions in this setting are incompletely understood. Here we find that mouse and human mast cells (MCs) express TNFRSF14 and that TNFSF14:TNFRSF14 interactions can enhance IgE-mediated MC signalling and mediator production. In mouse models of asthma, TNFRSF14 blockade with a neutralizing antibody administered after antigen sensitization, or genetic deletion of Tnfrsf14, diminishes plasma levels of antigen-specific IgG(1) and IgE antibodies, airway hyperreactivity, airway inflammation and airway remodelling. Finally, by analysing two types of genetically MC-deficient mice after engrafting MCs that either do or do not express TNFRSF14, we show that TNFRSF14 expression on MCs significantly contributes to the development of multiple features of asthma pathology.},
note = {Sibilano, Riccardo
Gaudenzio, Nicolas
DeGorter, Marianne K
Reber, Laurent L
Hernandez, Joseph D
Starkl, Philipp M
Zurek, Oliwia W
Tsai, Mindy
Zahner, Sonja
Montgomery, Stephen B
Roers, Axel
Kronenberg, Mitchell
Yu, Mang
Galli, Stephen J
T32 AI007290/AI/NIAID NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Nat Commun. 2016 Dec 16;7:13696. doi: 10.1038/ncomms13696.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Asthma has multiple features, including airway hyperreactivity, inflammation and remodelling. The TNF superfamily member TNFSF14 (LIGHT), via interactions with the receptor TNFRSF14 (HVEM), can support T(H)2 cell generation and longevity and promote airway remodelling in mouse models of asthma, but the mechanisms by which TNFSF14 functions in this setting are incompletely understood. Here we find that mouse and human mast cells (MCs) express TNFRSF14 and that TNFSF14:TNFRSF14 interactions can enhance IgE-mediated MC signalling and mediator production. In mouse models of asthma, TNFRSF14 blockade with a neutralizing antibody administered after antigen sensitization, or genetic deletion of Tnfrsf14, diminishes plasma levels of antigen-specific IgG(1) and IgE antibodies, airway hyperreactivity, airway inflammation and airway remodelling. Finally, by analysing two types of genetically MC-deficient mice after engrafting MCs that either do or do not express TNFRSF14, we show that TNFRSF14 expression on MCs significantly contributes to the development of multiple features of asthma pathology. |
Khazen, R.; Müller, S.; Gaudenzio, N.; Espinosa, E.; Puissegur, M. P.; Valitutti, S. Melanoma cell lysosome secretory burst neutralizes the CTL-mediated cytotoxicity at the lytic synapse Article de journal Dans: Nat Commun, vol. 7, p. 10823, 2016, (Khazen, Roxana
Müller, Sabina
Gaudenzio, Nicolas
Espinosa, Eric
Puissegur, Marie-Pierre
Valitutti, Salvatore
Research Support, Non-U.S. Gov't
Nat Commun. 2016 Mar 4;7:10823. doi: 10.1038/ncomms10823.). @article{d,
title = {Melanoma cell lysosome secretory burst neutralizes the CTL-mediated cytotoxicity at the lytic synapse},
author = {Khazen, R. and Müller, S. and Gaudenzio, N. and Espinosa, E. and Puissegur, M. P. and Valitutti, S.},
year = {2016},
date = {2016-01-01},
journal = {Nat Commun},
volume = {7},
pages = {10823},
abstract = {Human melanoma cells express various tumour antigens that are recognized by CD8(+) cytotoxic T lymphocytes (CTLs) and elicit tumour-specific responses in vivo. However, natural and therapeutically enhanced CTL responses in melanoma patients are of limited efficacy. The mechanisms underlying CTL effector phase failure when facing melanomas are still largely elusive. Here we show that, on conjugation with CTL, human melanoma cells undergo an active late endosome/lysosome trafficking, which is intensified at the lytic synapse and is paralleled by cathepsin-mediated perforin degradation and deficient granzyme B penetration. Abortion of SNAP-23-dependent lysosomal trafficking, pH perturbation or impairment of lysosomal proteolytic activity restores susceptibility to CTL attack. Inside the arsenal of melanoma cell strategies to escape immune surveillance, we identify a self-defence mechanism based on exacerbated lysosome secretion and perforin degradation at the lytic synapse. Interfering with this synaptic self-defence mechanism might be useful in potentiating CTL-mediated therapies in melanoma patients.},
note = {Khazen, Roxana
Müller, Sabina
Gaudenzio, Nicolas
Espinosa, Eric
Puissegur, Marie-Pierre
Valitutti, Salvatore
Research Support, Non-U.S. Gov't
Nat Commun. 2016 Mar 4;7:10823. doi: 10.1038/ncomms10823.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Human melanoma cells express various tumour antigens that are recognized by CD8(+) cytotoxic T lymphocytes (CTLs) and elicit tumour-specific responses in vivo. However, natural and therapeutically enhanced CTL responses in melanoma patients are of limited efficacy. The mechanisms underlying CTL effector phase failure when facing melanomas are still largely elusive. Here we show that, on conjugation with CTL, human melanoma cells undergo an active late endosome/lysosome trafficking, which is intensified at the lytic synapse and is paralleled by cathepsin-mediated perforin degradation and deficient granzyme B penetration. Abortion of SNAP-23-dependent lysosomal trafficking, pH perturbation or impairment of lysosomal proteolytic activity restores susceptibility to CTL attack. Inside the arsenal of melanoma cell strategies to escape immune surveillance, we identify a self-defence mechanism based on exacerbated lysosome secretion and perforin degradation at the lytic synapse. Interfering with this synaptic self-defence mechanism might be useful in potentiating CTL-mediated therapies in melanoma patients. |
Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L. L.; Cenac, N.; McNeil, B. D.; Dong, X.; Hernandez, J. D.; Sagi-Eisenberg, R.; Hammel, I.; Roers, A.; Valitutti, S.; Tsai, M.; Espinosa, E.; Galli, S. J. Different activation signals induce distinct mast cell degranulation strategies Article de journal Dans: J Clin Invest, vol. 126, no. 10, p. 3981-3998, 2016, (Gaudenzio, Nicolas
Sibilano, Riccardo
Marichal, Thomas
Starkl, Philipp
Reber, Laurent L
Cenac, Nicolas
McNeil, Benjamin D
Dong, Xinzhong
Hernandez, Joseph D
Sagi-Eisenberg, Ronit
Hammel, Ilan
Roers, Axel
Valitutti, Salvatore
Tsai, Mindy
Espinosa, Eric
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
P30 NS069375/NS/NINDS NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
S10 RR026780/RR/NCRR NIH HHS/United States
J Clin Invest. 2016 Oct 3;126(10):3981-3998. doi: 10.1172/JCI85538. Epub 2016 Sep 19.). @article{d,
title = {Different activation signals induce distinct mast cell degranulation strategies},
author = {Gaudenzio, N. and Sibilano, R. and Marichal, T. and Starkl, P. and Reber, L. L. and Cenac, N. and McNeil, B. D. and Dong, X. and Hernandez, J. D. and Sagi-Eisenberg, R. and Hammel, I. and Roers, A. and Valitutti, S. and Tsai, M. and Espinosa, E. and Galli, S. J.},
year = {2016},
date = {2016-01-01},
journal = {J Clin Invest},
volume = {126},
number = {10},
pages = {3981-3998},
abstract = {Mast cells (MCs) influence intercellular communication during inflammation by secreting cytoplasmic granules that contain diverse mediators. Here, we have demonstrated that MCs decode different activation stimuli into spatially and temporally distinct patterns of granule secretion. Certain signals, including substance P, the complement anaphylatoxins C3a and C5a, and endothelin 1, induced human MCs rapidly to secrete small and relatively spherical granule structures, a pattern consistent with the secretion of individual granules. Conversely, activating MCs with anti-IgE increased the time partition between signaling and secretion, which was associated with a period of sustained elevation of intracellular calcium and formation of larger and more heterogeneously shaped granule structures that underwent prolonged exteriorization. Pharmacological inhibition of IKK-β during IgE-dependent stimulation strongly reduced the time partition between signaling and secretion, inhibited SNAP23/STX4 complex formation, and switched the degranulation pattern into one that resembled degranulation induced by substance P. IgE-dependent and substance P-dependent activation in vivo also induced different patterns of mouse MC degranulation that were associated with distinct local and systemic pathophysiological responses. These findings show that cytoplasmic granule secretion from MCs that occurs in response to different activating stimuli can exhibit distinct dynamics and features that are associated with distinct patterns of MC-dependent inflammation.},
note = {Gaudenzio, Nicolas
Sibilano, Riccardo
Marichal, Thomas
Starkl, Philipp
Reber, Laurent L
Cenac, Nicolas
McNeil, Benjamin D
Dong, Xinzhong
Hernandez, Joseph D
Sagi-Eisenberg, Ronit
Hammel, Ilan
Roers, Axel
Valitutti, Salvatore
Tsai, Mindy
Espinosa, Eric
Galli, Stephen J
U19 AI104209/AI/NIAID NIH HHS/United States
P30 NS069375/NS/NINDS NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
UL1 RR025744/RR/NCRR NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
S10 RR026780/RR/NCRR NIH HHS/United States
J Clin Invest. 2016 Oct 3;126(10):3981-3998. doi: 10.1172/JCI85538. Epub 2016 Sep 19.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mast cells (MCs) influence intercellular communication during inflammation by secreting cytoplasmic granules that contain diverse mediators. Here, we have demonstrated that MCs decode different activation stimuli into spatially and temporally distinct patterns of granule secretion. Certain signals, including substance P, the complement anaphylatoxins C3a and C5a, and endothelin 1, induced human MCs rapidly to secrete small and relatively spherical granule structures, a pattern consistent with the secretion of individual granules. Conversely, activating MCs with anti-IgE increased the time partition between signaling and secretion, which was associated with a period of sustained elevation of intracellular calcium and formation of larger and more heterogeneously shaped granule structures that underwent prolonged exteriorization. Pharmacological inhibition of IKK-β during IgE-dependent stimulation strongly reduced the time partition between signaling and secretion, inhibited SNAP23/STX4 complex formation, and switched the degranulation pattern into one that resembled degranulation induced by substance P. IgE-dependent and substance P-dependent activation in vivo also induced different patterns of mouse MC degranulation that were associated with distinct local and systemic pathophysiological responses. These findings show that cytoplasmic granule secretion from MCs that occurs in response to different activating stimuli can exhibit distinct dynamics and features that are associated with distinct patterns of MC-dependent inflammation. |
Reber, L. L.; Gaudenzio, N.; Starkl, P.; Galli, S. J. Neutrophils are not required for resolution of acute gouty arthritis in mice Article de journal Dans: Nat Med, vol. 22, no. 12, p. 1382-1384, 2016, (Reber, Laurent L
Gaudenzio, Nicolas
Starkl, Philipp
Galli, Stephen J
K99 AI110645/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
U19 AI104209/AI/NIAID NIH HHS/United States
R21 NS080062/NS/NINDS NIH HHS/United States
Comment
Letter
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
United States
Nat Med. 2016 Dec 6;22(12):1382-1384. doi: 10.1038/nm.4216.). @article{d,
title = {Neutrophils are not required for resolution of acute gouty arthritis in mice},
author = {Reber, L. L. and Gaudenzio, N. and Starkl, P. and Galli, S. J.},
year = {2016},
date = {2016-01-01},
journal = {Nat Med},
volume = {22},
number = {12},
pages = {1382-1384},
note = {Reber, Laurent L
Gaudenzio, Nicolas
Starkl, Philipp
Galli, Stephen J
K99 AI110645/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
U19 AI104209/AI/NIAID NIH HHS/United States
R21 NS080062/NS/NINDS NIH HHS/United States
Comment
Letter
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
United States
Nat Med. 2016 Dec 6;22(12):1382-1384. doi: 10.1038/nm.4216.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Marichal, T.; Gaudenzio, N.; El Abbas, S.; Sibilano, R.; Zurek, O.; Starkl, P.; Reber, L. L.; Pirottin, D.; Kim, J.; Chambon, P.; Roers, A.; Antoine, N.; Kawakami, Y.; Kawakami, T.; Bureau, F.; Tam, S. Y.; Tsai, M.; Galli, S. J. Guanine nucleotide exchange factor RABGEF1 regulates keratinocyte-intrinsic signaling to maintain skin homeostasis Article de journal Dans: J Clin Invest, vol. 126, no. 12, p. 4497-4515, 2016, (Marichal, Thomas
Gaudenzio, Nicolas
El Abbas, Sophie
Sibilano, Riccardo
Zurek, Oliwia
Starkl, Philipp
Reber, Laurent L
Pirottin, Dimitri
Kim, Jinah
Chambon, Pierre
Roers, Axel
Antoine, Nadine
Kawakami, Yuko
Kawakami, Toshiaki
Bureau, Fabrice
Tam, See-Ying
Tsai, Mindy
Galli, Stephen J
T32 AI007290/AI/NIAID NIH HHS/United States
R01 HL124283/HL/NHLBI NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AR064418/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Clin Invest. 2016 Dec 1;126(12):4497-4515. doi: 10.1172/JCI86359. Epub 2016 Nov 7.). @article{d,
title = {Guanine nucleotide exchange factor RABGEF1 regulates keratinocyte-intrinsic signaling to maintain skin homeostasis},
author = {Marichal, T. and Gaudenzio, N. and El Abbas, S. and Sibilano, R. and Zurek, O. and Starkl, P. and Reber, L. L. and Pirottin, D. and Kim, J. and Chambon, P. and Roers, A. and Antoine, N. and Kawakami, Y. and Kawakami, T. and Bureau, F. and Tam, S. Y. and Tsai, M. and Galli, S. J.},
year = {2016},
date = {2016-01-01},
journal = {J Clin Invest},
volume = {126},
number = {12},
pages = {4497-4515},
abstract = {Epidermal keratinocytes form a structural and immune barrier that is essential for skin homeostasis. However, the mechanisms that regulate epidermal barrier function are incompletely understood. Here we have found that keratinocyte-specific deletion of the gene encoding RAB guanine nucleotide exchange factor 1 (RABGEF1, also known as RABEX-5) severely impairs epidermal barrier function in mice and induces an allergic cutaneous and systemic phenotype. RABGEF1-deficient keratinocytes exhibited aberrant activation of the intrinsic IL-1R/MYD88/NF-κB signaling pathway and MYD88-dependent abnormalities in expression of structural proteins that contribute to skin barrier function. Moreover, ablation of MYD88 signaling in RABGEF1-deficient keratinocytes or deletion of Il1r1 restored skin homeostasis and prevented development of skin inflammation. We further demonstrated that epidermal RABGEF1 expression is reduced in skin lesions of humans diagnosed with either atopic dermatitis or allergic contact dermatitis as well as in an inducible mouse model of allergic dermatitis. Our findings reveal a key role for RABGEF1 in dampening keratinocyte-intrinsic MYD88 signaling and sustaining epidermal barrier function in mice, and suggest that dysregulation of RABGEF1 expression may contribute to epidermal barrier dysfunction in allergic skin disorders in mice and humans. Thus, RABGEF1-mediated regulation of IL-1R/MYD88 signaling might represent a potential therapeutic target.},
note = {Marichal, Thomas
Gaudenzio, Nicolas
El Abbas, Sophie
Sibilano, Riccardo
Zurek, Oliwia
Starkl, Philipp
Reber, Laurent L
Pirottin, Dimitri
Kim, Jinah
Chambon, Pierre
Roers, Axel
Antoine, Nadine
Kawakami, Yuko
Kawakami, Toshiaki
Bureau, Fabrice
Tam, See-Ying
Tsai, Mindy
Galli, Stephen J
T32 AI007290/AI/NIAID NIH HHS/United States
R01 HL124283/HL/NHLBI NIH HHS/United States
R01 AI023990/AI/NIAID NIH HHS/United States
R01 AR067145/AR/NIAMS NIH HHS/United States
K99 AI110645/AI/NIAID NIH HHS/United States
R01 CA072074/CA/NCI NIH HHS/United States
R37 AI023990/AI/NIAID NIH HHS/United States
R01 AI070813/AI/NIAID NIH HHS/United States
R01 AR064418/AR/NIAMS NIH HHS/United States
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
J Clin Invest. 2016 Dec 1;126(12):4497-4515. doi: 10.1172/JCI86359. Epub 2016 Nov 7.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Epidermal keratinocytes form a structural and immune barrier that is essential for skin homeostasis. However, the mechanisms that regulate epidermal barrier function are incompletely understood. Here we have found that keratinocyte-specific deletion of the gene encoding RAB guanine nucleotide exchange factor 1 (RABGEF1, also known as RABEX-5) severely impairs epidermal barrier function in mice and induces an allergic cutaneous and systemic phenotype. RABGEF1-deficient keratinocytes exhibited aberrant activation of the intrinsic IL-1R/MYD88/NF-κB signaling pathway and MYD88-dependent abnormalities in expression of structural proteins that contribute to skin barrier function. Moreover, ablation of MYD88 signaling in RABGEF1-deficient keratinocytes or deletion of Il1r1 restored skin homeostasis and prevented development of skin inflammation. We further demonstrated that epidermal RABGEF1 expression is reduced in skin lesions of humans diagnosed with either atopic dermatitis or allergic contact dermatitis as well as in an inducible mouse model of allergic dermatitis. Our findings reveal a key role for RABGEF1 in dampening keratinocyte-intrinsic MYD88 signaling and sustaining epidermal barrier function in mice, and suggest that dysregulation of RABGEF1 expression may contribute to epidermal barrier dysfunction in allergic skin disorders in mice and humans. Thus, RABGEF1-mediated regulation of IL-1R/MYD88 signaling might represent a potential therapeutic target. |
0000
|
(No Title) Article de journal Dans: 0000. @article{d,
title = {(No Title)},
url = {http://science.sciencemag.org/},
doi = {10.1126/science.abf2022},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|