Eukaryotic Pathogens: Inflammation, T cell Immunity and Chemoresistance (EPIIC)
Responsable : N. Blanchard
Scientific objectives
N. Blanchard
Axis 1. Pathogenesis and neuroimmune responses during chronic Toxoplasma gondii infection
F. Masson
Axis 2. Transcriptional regulation of T cell differentiation during neuroinflammatory diseases
X. Iriart
Axis 3. Pathophysiology of the adaptive immune response and involvement of inflammation during Pneumocystis infection
A. Berry
Axis 4: Monitor the spreading of drug-resistant malaria parasites and analyze the molecular mechanisms of resistance
Eukaryotic pathogens affect the lives of millions of individuals throughout the world. These pathogens include fungi such as Pneumocystis and intracellular parasites such as Toxoplasma gondii and Plasmodium spp.
Pneumocystis pneumonia is an opportunistic fungal disease which induces life-threatening respiratory distress syndrome, and a frequent cause of pneumopathy among immunocompromised patients with a high mortality rate.
Toxoplasmosis is also an opportunistic disease. It is caused by T. gondii infection and may lead to chorioretinitis or encephalitis in immunocompromised individuals, and fetal abnormalities if contracted during pregnancy. Even in immunocompetent individuals, latent T. gondii infection has a number of neurological consequences which may have been underestimated so far.
Malaria is the disease caused by Plasmodium infection. Malaria kills almost half a million people every year, with the biggest toll on children who suffer from deadly complications like cerebral malaria.
Our research addresses how immunity develops in response to these eukaryotic pathogens. We are trying to understand the mechanisms by which T cells differentiate and control these pathogens, and how the pathogens may modulate the T cell responses to their benefits. We also analyze the processes induced by infection that may lead to excessive reactions and immunopathology. At last, we work on the epidemiology and mechanisms of drug resistance of malaria parasites.
Our Projects
AXIS 1. Pathogenesis and neuroimmune responses during chronic Toxoplasma gondii infection
The Toxoplasma gondii (T. gondii) parasite is a food-borne pathogen that is widespread in the environment. Its reservoirs are infected animals and contaminated water and soil. It is estimated that 30% of the population worldwide (up to 50% in France) have been exposed to T. gondii (Fig. 1).
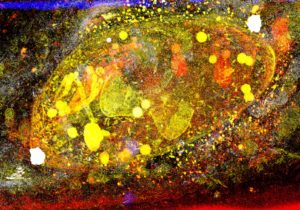
Fig. 1. A Toxoplasma tachyzoite within its vacuole. Pseudo-colored artwork of a Focused Ion Beam-Scanning Electron Microscopy (FIB-SEM) stack projected onto one plane. Credits: J. Santi-Rocca
After ingestion of contaminated products, the parasites multiply and reach all tissues of the host including the brain. Immune reactions are elicited but they are unable to clear the parasite. T. gondii then establishes latency in cysts that reside in muscular cells and neurons (Fig. 2)
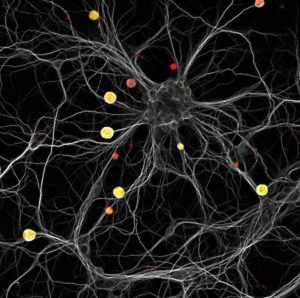
Fig. 2. A constellation of yellow cysts in a neuronal galaxy. This image shows a mouse hippocampal neuronal culture infected with red fluorescent Toxoplasma tachyzoites. Three days post-infection, the culture was labeled with a neuronal (white) and a cyst (green) marker. Cysts can be visualized as yellow structures scattered within the neuronal dendritic neuronal network. Credits: M. Belloy & A. Lebourg
In the central nervous system (CNS), T. gondii is under continuous immune surveillance, which avoids parasite reactivation and brain damage. This surveillance is achieved in large part by parasite-specific CD8+ T cells that detect antigenic fragments from T. gondii presented by MHC I molecules at the surface of infected neurons (Salvioni et al., Cell Reports 2019). Interestingly, even when efficiently controlled, latent T. gondii infection is associated with multiple neurological disorders, which are thought to be, at least in part, due to neuroinflammation (Laing et al., Trends Immunol 2020).
We are keen to understand the role of the neuron-T cell crosstalk in host immune protection and in the perturbations of neuronal functions induced by the parasite. Moreover, we have a strong interest in unraveling the consequences of parasite latency outside of the CNS, i.e. on the peripheral (enteric) nervous system.
Our previous work has allowed us to design unique models to study the neuroimmune crosstalk (Fig. 3).
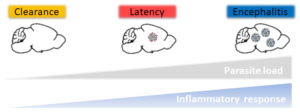 Fig. 3. Three in vivo models to study Toxoplasma infection. Depending on the immunodominant antigen expressed by the parasites (GRA6-OVA vs. SAG1-OVA) and the timing of administration of the pyrimethamine anti-parasite drug, the outcome of infection varies from clearance to latency to encephalitis, thereby recapitulating the different contexts of human infection.
Fig. 3. Three in vivo models to study Toxoplasma infection. Depending on the immunodominant antigen expressed by the parasites (GRA6-OVA vs. SAG1-OVA) and the timing of administration of the pyrimethamine anti-parasite drug, the outcome of infection varies from clearance to latency to encephalitis, thereby recapitulating the different contexts of human infection.
These models recapitulate 3 situations that are observed in humans: latency (most frequent), encephalitis (may occur in case of acquired immune deficiency), clearance (may occur in case of prompt anti-parasitic drug treatment). We are now studying:
1- how the neuroimmune crosstalk controls behavioral changes and neurodegeneration during T. gondii infection
Project leader : M. Belloy (PhD student)
Collaboration : Dr E. Suberbielle and Dr N. Fazilleau, Infinity, Toulouse
2- how the neuroimmune crosstalk controls T. gondii-induced changes in intestinal homestasis and visceral sensitivity (Audibert et al, PLoS Pathogens 2025)
Project leader : A. Audibert (PhD student)
Collaboration : Dr C. Bonnart, Dr G. Dietrich and Dr N. Cenac, IRSD, Toulouse
3- the function(s) and mechanisms of differentiation/maintenance of brain-resident memory CD8+ T cells during chronic T. gondii infection (Porte et al, PNAS 2024)
Project leaders : R. Porte (postdoc),
Collaboration : Dr K. van Gisbergen, Sanquin Institute, The Netherlands ; Dr F. Masson, Infinity
4- how T. gondii cysts evade CD8 T cell recognition to establish latency
Project leader : R. Gutierrez-Loli (PhD student)
Collaboration : Dr M.-A. Hakimi, IAB, Grenoble
Given that T. gondii cysts cannot be eradicated pharmacologically, CD8+ T cells represent valuable cellular targets to harness in order to eliminate the parasite from latently infected hosts.
Axis 2. Transcriptional regulation of T cell differentiation during neuroinflammatory diseases
We are interested by the molecular mechanisms that regulate the functional specialization of T lymphocytes during neuroinflammatory diseases caused by a pathogen infection (in particular the infection by the intracellular parasite Toxoplasma gondii) or during CNS auto-immunity (multiple sclerosis).
Specifically, we are investigating the mechanisms by which key transcriptional regulators control the differentiation of T cells into effector and memory cells (Fig. 1), as well their roles in these various pathophysiological contexts, which may be either protective or pathogenic.
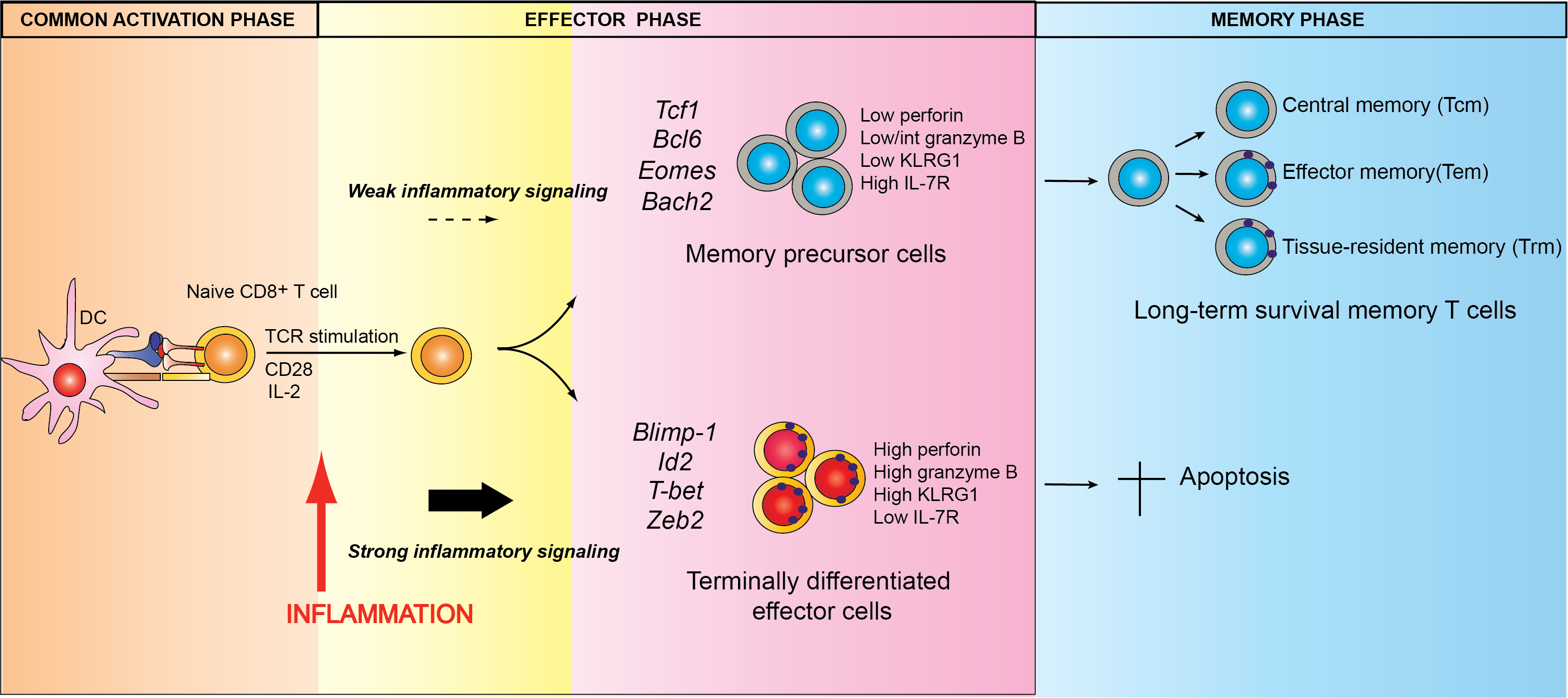
Fig. 1. Model of peripheral CD8+ T cell differentiation during a pathogen infection. Credits F. Masson
We are using in vivo experimental models of neuroinflammation (infection by T. gondii, experimental autoimmune encephalitis (EAE)) associated with state-of-the-art molecular tools including inducible lentiviral and retroviral vectors allowing genetic alteration of T cells in an inducible manner.
Our projects aim at identifying new therapeutic strategies that are more targeted and efficacious against neuroinflammatory diseases through the functional reprogramming of T lymphocytes.
Axis 3. Pathophysiology of the adaptive immune response and involvement of inflammation during Pneumocystis infection
Pneumocystis pneumonia (PCP) is an opportunistic fungal disease which induces potentially life-threatening respiratory distress syndrome. While immunocompetent hosts spontaneously eliminate the fungus (Fig. 1) without any symptoms, PCP is a frequent cause of pneumopathy among immunocompromised patients, with a mortality estimated at ~20%. Yet, PCP and the associated immune responses are understudied.
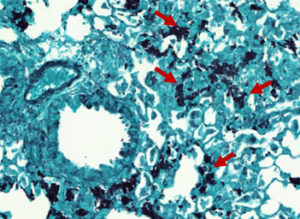
Fig. 1. Pneumocystosis in an in vivo experimental model infected with Pneumocystis murina: Pneumocystis cysts stained with Gomori Grocott (red arrows)
Our group addresses 2 specific aims on the topic of Pneumocystis infection:
- A better understanding of the pathophysiological mechanisms of PCP, particularly adaptive immunity and host inflammatory response
PCP pathophysiology remains poorly understood. However, it is generally accepted that the lung lesions observed during the disease are largely due to an unsuitable host immune response (Fig. 2). We previously identified pro-inflammatory and pro-resolving lipid mediators which seem to be implicated in the pathophysiology of the disease both in PCP patients and in in vivo experimental models of Pneumocystis infection.
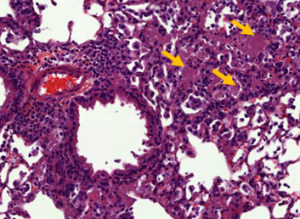
Fig. 2. Pneumocystosis in an in vivo experimental model infected with Pneumocystis murina: Alveolar exudates after staining with Hematoxylin-Eosin (yellow arrows)
As the lymphocyte response is crucial in the disease development, we are investigating the implication of the different CD4+ T lymphocyte subsets in the pathophysiology of Pneumocystis infection and their potential as key effector cells to control PCP pathology.
- The definition of biomarkers predictive of the risk or the outcome of PCP
Identifying biological markers predictive of a high PCP risk or outcome is essential for the clinical management of immunocompromised patients. Thanks to clinical studies, we have already identified lymphocyte subsets and pro-inflammatory lipid mediators associated with unfavourable evolution of PCP.
Prophylactic treatments are effective in preventing the development of PCP in immunocompromised patients. Nevertheless, there is no good biomarker of PCP risk for the HIV-negative population. Using in vivo experimental models of PCP, we identified lymphocyte subsets which seem to be associated with Pneumocystis elimination in immunocompetent subjects without notable inflammation.
Using clinical studies and in vivo experimental models of PCP, our project aims at evaluating the potential interest of these populations as biomarkers of PCP risk, particularly in immunocompromised HIV-negative patients.
Axis 4: Monitor the spreading of drug-resistant malaria parasites and analyze the molecular mechanisms of resistance
Regular monitoring of the levels of anti-malarial resistance of P. falciparum is an essential policy to adapt therapy and ensure effective malaria control. In collaboration with the Institute for Research and Development and the Pasteur Center of Cameroon, we assess the impact of anti-malarial treatments on the emergence and spread of resistant parasites in Cameroon (Ménard et al, Malar J 2012 and Malar J. 2016; Chauvin et al, J Antimicrob Chemother 2015).
Artemisinin-derivatives (ART) are the last still effective anti-malarial class but emergence of resistant P. falciparum first identified in Southeast Asia and recently in Africa, is a dramatic public health concern. In collaboration with F. Benoit-Vical (LCC CNRS, Toulouse), then with the Pasteur Institutes of Paris and Cambodia, we have demonstrated that a strain highly resistant to ART in vitro, survives toxic effects of ART through temporary growth arrest (quiescence), as has been reported in malaria isolates from patients in Asia (Witkowski et al, AAC 2010 and AAC 2013). These findings allowed the identification of the first gene strongly associated with ART-resistance (Ariey et al, Nature 2014).
Based on this work, we are addressing 2 specific aims:
- Monitor the emergence and spread of P. falciparum chemoresistance in Africa.
2. Characterize the mechanisms underlying quiescence of ART-resistant P. falciparum.

A quiescent Plasmodium falciparum parasite in a human erythrocyte following artemisinin treatment

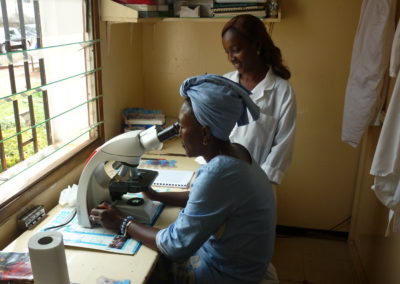
OCEAC, Organisation de Coordination et de Coopération pour la lutte contre les grandes Endémies en Afrique Centrale (Yaoundé, Cameroun).
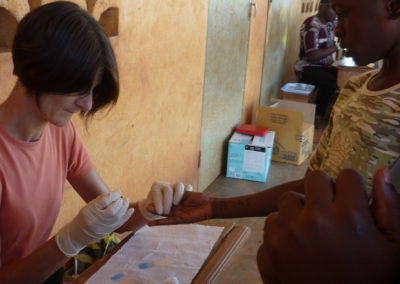
Screening children for malaria infection in the Mfou district (Cameroun)
Other details
Our team
Publications
2025 |
Audibert, Alexis; Mas-Orea, Xavier; Rey, Léa; Belloy, Marcy; Bassot, Emilie; Battut, Louise; Marodon, Gilles; Masson, Frederick; Serino, Matteo; Cenac, Nicolas; Dietrich, Gilles; Bonnart, Chrystelle; Blanchard, Nicolas Toxoplasma gondii chronic infection decreases visceral nociception through peripheral opioid receptor signaling Journal Article In: PLoS Pathog, vol. 21, no. 4, pp. e1013106, 2025, ISSN: 1553-7374. @article{pmid40300021,By eliciting immune activation in the digestive tract, intestinal pathogens may perturb gut homeostasis. Some gastrointestinal infections can indeed increase the risk of developing post-infectious irritable bowel syndrome (PI-IBS). Intriguingly, the prevalent foodborne parasite Toxoplasma gondii has not been linked to the development of PI-IBS and the impact of this infection on colon homeostasis remains ill-defined. We show in a mouse model that latent T. gondii decreases visceral nociceptive responses in an opioid signaling-dependent manner. Despite the accumulation of Th1 and cytotoxic T cells in the colon of latently infected mice, the selective invalidation of enkephalin gene in T cells ruled out the involvement of T cell-derived enkephalins in hypoalgesia. These findings provide clues about how this widespread infection durably shapes the gut immune landscape and modifies intestinal physiological parameters. They suggest that in contrast to other gut microbes, T. gondii infection could be negatively associated with abdominal pain. |
Cebrian, Ignacio; Dinamarca, Sofía; Rodríguez, María Jesús Pena; Priego, Elena; Brouwers, Nathalie; Barends, Martina; Brunnberg, Jamina; Tampé, Robert; Blanchard, Nicolas; Sancho, David; Malhotra, Vivek Dendritic cell phagosomes recruit GRASP55 for export of antigen-loaded MHC molecules Journal Article In: Cell Rep, vol. 44, no. 2, pp. 115333, 2025, ISSN: 2211-1247. @article{pmid39955774,Dendritic cells (DCs) present exogenous antigens via major histocompatibility complex class I (MHC-I) and MHC class II (MHC-II) molecules, activating CD8 and CD4 T cells. A critical but poorly understood step in this process is the trafficking of peptide-loaded MHC molecules from the endocytic system to the cell surface. In this study, we demonstrate that the Golgi reassembly-stacking protein of 55 kDa (GRASP55), which has been shown to have no role in stacking, is essential for antigen presentation. Using soluble, bead-coated, and bacterial-bound antigens, we found significantly impaired exogenous antigen presentation in GRASP55-deficient bone-marrow-derived DCs (BMDCs). Notably, GRASP55 was recruited to late phagosomes, and our data suggest that it is crucial for sorting MHC-I and MHC-II molecules, facilitating their trafficking to the plasma membrane. Our findings highlight the vital role of GRASP55 in the intracellular transport of MHC molecules bound to their respective peptides during exogenous antigen presentation. |
Dinamarca, Sofía; Croce, Cristina; Salvioni, Anna; Garrido, Facundo; Fidalgo, Sandra Estrada; Bigliani, Gonzalo; Mayorga, Luis S; Blanchard, Nicolas; Cebrian, Ignacio SNX17 Regulates Antigen Internalisation and Phagosomal Maturation by Dendritic Cells Journal Article In: Immunology, vol. 174, no. 1, pp. 167–185, 2025, ISSN: 1365-2567. @article{pmid39559950,Antigen cross-presentation is the process whereby small peptides derived from exogenous antigens are attached to MHC-I molecules triggering CD8+ T lymphocyte activation. The endocytic route of dendritic cells (DCs) is highly specialised for cross-presentation to initiate cytotoxic immune responses against numerous intracellular pathogens and tumours. In this study, we identify the endosomal protein sorting nexin (SNX) 17 as a key regulator of antigen internalisation and cross-presentation by DCs. SNX17 expression in DCs guarantees optimal cross-presentation of soluble, particulate, and Toxoplasma gondii-associated antigens. The silencing of SNX17 expression in DCs significantly affected the internalisation of exogenous antigens by fluid-phase endocytosis, phagocytosis, and more strikingly, T. gondii invasion. We show that SNX17 controls proper integrin recycling, actin cytoskeleton organisation, and phagosomal maturation. Altogether, our findings provide compelling evidence that SNX17 plays a central role in the modulation of the DC endocytic network, which is essential for competent antigen cross-presentation. |
2024 |
Aubert, Nicolas; Purcarea, Madeleine; Novarino, Julien; Schopp, Julien; Audibert, Alexis; Li, Wangtianrui; Fornier, Marie; Cagnet, Léonie; Naturel, Marie; Casrouge, Armanda; Dieu-Nosjean, Marie-Caroline; Blanchard, Nicolas; Dietrich, Gilles; Peirs, Cedric; Marodon, Gilles Enkephalin-mediated modulation of basal somatic sensitivity by regulatory T cells in mice Journal Article In: Elife, vol. 13, 2024, ISSN: 2050-084X. @article{pmid39110619,CD4CD25Foxp3 regulatory T cells (Treg) have been implicated in pain modulation in various inflammatory conditions. However, whether Treg cells hamper pain at steady state and by which mechanism is still unclear. From a meta-analysis of the transcriptomes of murine Treg and conventional T cells (Tconv), we observe that the proenkephalin gene (), encoding the precursor of analgesic opioid peptides, ranks among the top 25 genes most enriched in Treg cells. We then present various evidence suggesting that is regulated in part by members of the Tumor Necrosis Factor Receptor (TNFR) family and the transcription factor Basic leucine zipper transcription faatf-like (BATF). Using mice in which the promoter activity of can be tracked with a fluorescent reporter, we also show that expression is mostly detected in Treg and activated Tconv in non-inflammatory conditions in the colon and skin. Functionally, Treg cells proficient or deficient for suppress equally well the proliferation of effector T cells in vitro and autoimmune colitis in vivo. In contrast, inducible ablation of in Treg leads to heat hyperalgesia in both male and female mice. Overall, our results indicate that Treg might play a key role at modulating basal somatic sensitivity in mice through the production of analgesic opioid peptides. |
Porte, Rémi; Belloy, Marcy; Audibert, Alexis; Bassot, Emilie; Aïda, Amel; Alis, Marine; Miranda-Capet, Romain; Jourdes, Aurélie; van Gisbergen, Klaas P J M; Masson, Frédérick; Blanchard, Nicolas Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent infection Journal Article In: Proc Natl Acad Sci U S A, vol. 121, no. 24, pp. e2403054121, 2024, ISSN: 1091-6490. @article{pmid38838017b,Chronic infection induces brain-resident CD8+ T cells (bTr), but the protective functions and differentiation cues of these cells remain undefined. Here, we used a mouse model of latent infection by leading to effective CD8+ T cell-mediated parasite control. Thanks to antibody depletion approaches, we found that peripheral circulating CD8+ T cells are dispensable for brain parasite control during chronic stage, indicating that CD8+ bTr are able to prevent brain parasite reactivation. We observed that the retention markers CD69, CD49a, and CD103 are sequentially acquired by brain parasite-specific CD8+ T cells throughout infection and that a majority of CD69/CD49a/CD103 triple-positive (TP) CD8+ T cells also express Hobit, a transcription factor associated with tissue residency. This TP subset develops in a CD4+ T cell-dependent manner and is associated with effective parasite control during chronic stage. Conditional invalidation of Transporter associated with Antigen Processing (TAP)-mediated major histocompatibility complex (MHC) class I presentation showed that presentation of parasite antigens by glutamatergic neurons and microglia regulates the differentiation of CD8+ bTr into TP cells. Single-cell transcriptomic analyses revealed that resistance to encephalitis is associated with the expansion of stem-like subsets of CD8+ bTr. In summary, parasite-specific brain-resident CD8+ T cells are a functionally heterogeneous compartment which autonomously ensure parasite control during latent infection and which differentiation is shaped by neuronal and microglial MHC I presentation. A more detailed understanding of local T cell-mediated immune surveillance of this common parasite is needed for harnessing brain-resident CD8+ T cells in order to enhance control of chronic brain infections. |
2023 |
Guemas, E.; Coppee, R.; Menard, S.; du Manoir, M.; Nsango, S.; Makaba Mvumbi, D.; Nakoune, E.; Eboumbou Moukoko, C. E.; Bouyou Akotet, M. K.; Mirabeau, T. Y.; Manguin, S.; Malekita Yobi, D.; Akiana, J.; Kouna, L. C.; Mawili Mboumba, D. P.; Voumbo-Matoumona, D. F.; Otam, A. L.; Rubbo, P. A.; Lombart, J. P.; Kwanai, E.; Cohen, O.; Iriart, X.; Ayong, L.; Lekana-Douki, J. B.; Ariey, F.; Berry, A. Evolution and spread of Plasmodium falciparum mutations associated with resistance to sulfadoxine-pyrimethamine in central Africa: a cross-sectional study Journal Article In: Lancet Microbe, 2023, (doi: 10.1016/S2666-5247(23)00211-2.). @article{c,BACKGROUND: Efficacy of sulfadoxine-pyrimethamine, the malaria chemoprophylaxis used in pregnant women, and in children when combined with amodiaquine, is threatened by the accumulation of mutations in the Plasmodium falciparum dihydropteroate synthase (pfdhps) and dihydrofolate reductase (pfdhfr) genes. Data on the prevalence of resistant alleles in central Africa and the new pfdhps I431V mutation, particularly associated with other mutations to form the pfdhps vagKgs allele, are scarce. We explored the frequency and geographical distribution of pfdhps and pfdhfr mutations in central Africa in 2014-18, and assessed the evolutionary origin of the vagKgs allele. METHODS: Samples were collected at 18 health-care centres in seven countries (Angola, Cameroon, Central African Republic, Democratic Republic of the Congo, Gabon, Nigeria, and Republic of the Congo) from patients who showed possible symptoms of malaria between March 1, 2014, and Oct 31, 2018. Samples that were positive for P falciparum were transported to a laboratory in Toulouse, France, and genotyped. The frequency of pfdhfr and pfdhps mutations was studied in 1749 samples. Microsatellites in pfdhps flanking regions and whole-genome analysis compared with parasite genomes from the data-sharing network MalariaGEN were performed on samples carrying the vagKgs allele. FINDINGS: Mapping of the prevalence of single nucleotide polymorphisms and corresponding alleles of pfdhfr and pfdhps showed a substantial spread of alleles associated with sulfadoxine-pyrimethamine resistance in central Africa during the 2014-18 period, especially an increase going west to east in pfdhps alleles carrying the K540E and A581G mutations. A high prevalence of the pfdhps I431V mutation was observed in Cameroon (exceeding 50% in the northern region) and Nigeria. Genomic analysis showed a recent African emergence and a clonal expansion of the most frequent pfdhps vagKgs allele. INTERPRETATION: Reduced sulfadoxine-pyrimethamine efficacy due to increased resistance is a worrying situation, especially because the malaria transmission level is high in central Africa. Although the resistance phenotype remains to be confirmed, the emergence and spread of the vagKgs allele in west and central Africa could challenge the use of sulfadoxine-pyrimethamine. FUNDING: Toulouse Institute for Infectious and Inflammatory Diseases. |
Cohen, O.; Guemas, E.; Menard, S.; Tsague Kenfack, M.; Talom Ngassa, C.; Iriart, X.; Bidzogo Lebobo, M.; Ondobo Ekae, C.; Eboumbou, C.; Tiyou Kenmeni, C.; Berry, A. Effect of sulfadoxine-pyrimethamine chemoprophylaxis in pregnant women on selection of the new P. falciparum dhps quintuple mutant carrying the I431V mutation Journal Article In: J Antimicrob Chemother, vol. 78, no. 3, pp. 665-668, 2023, ( doi: 10.1093/jac/dkac432.). @article{c,BACKGROUND: A new mutation in the Plasmodium falciparum dihydropteroate synthetase gene (pfdhps), I431V, has been identified in several countries of Central and West Africa. This mutation is mostly found in association with four other SNPs on pfdhps (S436A, A437G, A581G and A613S), forming a quintuple mutant (vagKgs) and almost always associated with the Plasmodium falciparum dihydrofolate reductase gene (pfdhfr) CirnI (C50R, N51I, S108N) triple mutant. To date, nothing is known about the impact of this new pfdhps genotype on sulfadoxine-pyrimethamine (SP) resistance. OBJECTIVES: We sought to assess the prevalence of this pfdhps vagKgs quintuple mutant in two groups of pregnant women with malaria, one that took intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) and one that did not. METHODS: The pfdhfr and pfdhps genes from Plasmodium falciparum isolates collected in Yaounde (Cameroon) from pregnant women with symptomatic malaria under IPTp-SP or not, were sequenced. RESULTS: Of 159 patients evaluated, 70 had already taken SP during pregnancy and 89 had never taken SP. Only the vagKgs allele was significantly overrepresented in the SP+ group (21.4% versus 3.4%; P < 0.001), whereas the ISgKAA mutant, widely distributed in this area and known to be less susceptible to SP, tended to be less abundant in this group (48.6% versus 64.0%; P = 0.0503). CONCLUSIONS: We found a strong overrepresentation of the CirnI/vagKgs haplotype in the IPTp-SP pregnant group, suggesting a high level of resistance of this mutant to SP. This could compromise not only the effectiveness of IPTp-SP but also the seasonal malaria chemoprevention of young children, now widely implemented. |
Sanchez, S. G.; Bassot, E.; Cerutti, A.; Mai Nguyen, H.; Aida, A.; Blanchard, N.; Besteiro, S. The apicoplast is important for the viability and persistence of Toxoplasma gondii bradyzoites Journal Article In: Proc Natl Acad Sci U S A, vol. 120, no. 34, pp. e2309043120, 2023, (doi:10.1073/pnas.2309043120). @article{c,Toxoplasma gondii is responsible for toxoplasmosis, a disease that can be serious when contracted during pregnancy, but can also be a threat for immunocompromised individuals. Acute infection is associated with the tachyzoite form that spreads rapidly within the host. However, under stress conditions, some parasites can differentiate into cyst-forming bradyzoites, residing mainly in the central nervous system, retina and muscle. Because this latent form of the parasite is resistant to all currently available treatments, and is central to persistence and transmission of the parasite, specific therapeutic strategies targeting this developmental stage need to be found. T. gondii contains a plastid of endosymbiotic origin called the apicoplast, which is an appealing drug target because it is essential for tachyzoite viability and contains several key metabolic pathways that are largely absent from the mammalian host. Its function in bradyzoites, however, is unknown. Our objective was thus to study the contribution of the apicoplast to the viability and persistence of bradyzoites during chronic toxoplasmosis. We have used complementary strategies based on stage-specific promoters to generate conditional bradyzoite mutants of essential apicoplast genes. Our results show that specifically targeting the apicoplast in both in vitro or in vivo-differentiated bradyzoites leads to a loss of long-term bradyzoite viability, highlighting the importance of this organelle for this developmental stage. This validates the apicoplast as a potential area to look for therapeutic targets in bradyzoites, with the aim to interfere with this currently incurable parasite stage. |
2022 |
Croce, C.; Garrido, F.; Dinamarca, S.; Santi-Rocca, J.; Marion, S.; Blanchard, N.; Mayorga, L. S.; Cebrian, I. Efficient Cholesterol Transport in Dendritic Cells Defines Optimal Exogenous Antigen Presentation and Toxoplasma gondii Proliferation Journal Article In: Front Cell Dev Biol, vol. 10, pp. 837574, 2022, (doi: 10.3389/fcell.2022.837574. eCollection 2022.). @article{c,Dendritic cells are the most powerful antigen-presenting cells of the immune system. They present exogenous antigens associated with Major Histocompatibility Complex (MHC) Class II molecules through the classical pathway to stimulate CD4+ T cells, or with MHC-I to activate CD8+ T lymphocytes through the cross-presentation pathway. DCs represent one of the main cellular targets during infection by Toxoplasma gondii. This intracellular parasite incorporates essential nutrients, such as cholesterol, to grow and proliferate inside a highly specialized organelle, the parasitophorous vacuole (PV). While doing so, T. gondii modulates the host immune response through multiple interactions with proteins and lipids. Cholesterol is an important cellular component that regulates cellular physiology at the structural and functional levels. Although different studies describe the relevance of cholesterol transport for exogenous antigen presentation, the molecular mechanism underlying this process is not defined. Here, we focus our study on the inhibitor U18666A, a drug widely used to arrest multivesicular bodies biogenesis that interrupts cholesterol trafficking and changes the lipid composition of intracellular membranes. Upon bone marrow-derived DC (BMDC) treatment with U18666A, we evidenced a drastic disruption in the ability to present exogenous soluble and particulate antigens to CD4+ and CD8+ T cells. Strikingly, the presentation of T. gondii-associated antigens and parasite proliferation were hampered in treated cells. However, neither antigen uptake nor BMDC viability was significantly affected by the U18666A treatment. By contrast, this drug altered the transport of MHC-I and MHC-II molecules to the plasma membrane. Since U18666A impairs the formation of MVBs, we analyzed in T. gondii infected BMDCs the ESCRT machinery responsible for the generation of intraluminal vesicles. We observed that different MVBs markers, including ESCRT proteins, were recruited to the PV. Surprisingly, the main ESCRT-III component CHMP4b was massively recruited to the PV, and its expression level was upregulated upon BMDC infection by T. gondii. Finally, we demonstrated that BMDC treatment with U18666A interrupted cholesterol delivery and CHMP4b recruitment to the PV, which interfered with an efficient parasite replication. Altogether, our results highlight the importance of cholesterol trafficking and MVBs formation in DCs for optimal antigen presentation and T. gondii proliferation. |
Frieser, D.; Pignata, A.; Khajavi, L.; Shlesinger, D.; Gonzalez-Fierro, C.; Nguyen, X. H.; Yermanos, A.; Merkler, D.; Hoftberger, R.; Desestret, V.; Mair, K. M.; Bauer, J.; Masson, F.; Liblau, R. S. Tissue-resident CD8(+) T cells drive compartmentalized and chronic autoimmune damage against CNS neurons Journal Article In: Sci Transl Med, vol. 14, no. 640, pp. eabl6157, 2022, (doi: 10.1126/scitranslmed.abl6157. Epub 2022 Apr 13.). @article{c,The mechanisms underlying the chronicity of autoimmune diseases of the central nervous system (CNS) are largely unknown. In particular, it is unclear whether tissue-resident memory T cells (T(RM)) contribute to lesion pathogenesis during chronic CNS autoimmunity. Here, we observed that a high frequency of brain-infiltrating CD8(+) T cells exhibit a T(RM)-like phenotype in human autoimmune encephalitis. Using mouse models of neuronal autoimmunity and a combination of T single-cell transcriptomics, high-dimensional flow cytometry, and histopathology, we found that pathogenic CD8(+) T cells behind the blood-brain barrier adopt a characteristic T(RM) differentiation program, and we revealed their phenotypic and functional heterogeneity. In the diseased CNS, autoreactive tissue-resident CD8(+) T cells sustained focal neuroinflammation and progressive loss of neurons, independently of recirculating CD8(+) T cells. Consistently, a large fraction of autoreactive tissue-resident CD8(+) T cells exhibited proliferative potential as well as proinflammatory and cytotoxic properties. Persistence of tissue-resident CD8(+) T cells in the CNS and their functional output, but not their initial differentiation, were crucially dependent on CD4(+) T cells. Collectively, our results point to tissue-resident CD8(+) T cells as essential drivers of chronic CNS autoimmunity and suggest that therapies targeting this compartmentalized autoreactive T cell subset might be effective for treating CNS autoimmune diseases. |
Guemas, E.; Cassaing, S.; Malavaud, S.; Fillaux, J.; Chauvin, P.; Lelievre, L.; Ruiz, S.; Riu, B.; Berry, A.; Iriart, X. A Clustered Case Series of Mucorales Detection in Respiratory Samples from COVID-19 Patients in Intensive Care, France, August to September 2021 Journal Article In: J Fungi (Basel), vol. 8, no. 3, 2022, (doi: 10.3390/jof8030258.). @article{c,While COVID-19-associated pulmonary aspergillosis is now well described in developed countries, COVID-19-associated mucormycosis (CAM) has seemed to remain quite rare in Europe. A retrospective study was performed between March 2020 to September 2021 among COVID-19 adult patients in the intensive care unit (ICU) at Toulouse Hospital (Southern France). PCR screening on respiratory samples, which target Aspergillus or Mucorales DNA, were performed, and the number of fungal detections was evaluated monthly during the study period. During the 19 months of the study, 44 (20.3%) COVID-19 ICU patients had a positive PCR for Aspergillus, an overall rate in keeping with the incidence of ICU COVID-19 patients. Ten patients (7.1%) had a positive Mucorales PCR over the same period. Surprisingly, 9/10 had a positive Mucor/Rhizopus PCR in August-September 2021, during the fourth Delta SARS-CoV-2 variant wave. Epidemic investigations have identified a probable environmental cause linked to construction works in the vicinity of the ICU (high levels of airborne spores due to the mistaken interruption of preventive humidification and summer temperature). Even if CAM are apparently rare in Europe, a cluster can also develop in industrialised countries when environmental conditions (especially during construction work) are associated with a high number of COVID-19 patients in the ICU. |
Merkler, D.; Vincenti, I.; Masson, F.; Liblau, R. S. Tissue-resident CD8 T cells in central nervous system inflammatory diseases: present at the crime scene and ...guilty Journal Article In: Curr Opin Immunol, vol. 77, pp. 102211, 2022, (doi: 10.1016/j.coi.2022.102211. Epub 2022 May 26.). @article{c,Tissue-resident memory T cells (T(RM)) represent a subset of antigen-experienced T cells that are constantly retained in a given tissue with limited trafficking through the circulation. These cells are characterized by expression of molecules enabling their tissue anchoring and downregulation of molecules promoting tissue egress. They reside at sites of previous antigen encounter and their number increases with age. T(RM) have been shown to provide rapid and efficient protection against tissue reinfection and T(RM) density correlates with efficient antitumor responses. Intriguingly, the density of CD8 T(RM) is increased in the central nervous system (CNS) of patients with neuroinflammatory diseases such as multiple sclerosis, or suffering from neurodegenerative diseases. In this review, we discuss current knowledge regarding the diversity of CNS-resident CD8 T cells and their role in CNS autoimmunity. Given their likely contribution to the protracted course of several inflammatory diseases of the CNS, their therapeutic targeting becomes an important challenge. |
Sarah-Matio, E. M.; Guillochon, E.; Nsango, S. E.; Abate, L.; Ngou, C. M.; Bouopda, G. A.; Feufack-Donfack, L. B.; Bayibeki, A. N.; Tchioffo Tsapi, M.; Talman, A.; Marin-Menendez, A.; Ayong, L.; Claessens, A.; Lefevre, T.; Berry, A.; Morlais, I. Genetic Diversity of Plasmodium falciparum and Distribution of Antimalarial Drug Resistance Mutations in Symptomatic and Asymptomatic Infections Journal Article In: Antimicrob Agents Chemother, vol. 66, no. 8, pp. e0018822, 2022, (doi: 10.1128/aac.00188-22. Epub 2022 Jul 6.). @article{c,Malaria control relies on passive case detection, and this strategy fails detecting asymptomatic infections. In addition, infections in endemic areas harbor multiple parasite genotypes that could affect case management and malaria epidemiology. Here, we performed AmpSeq genotyping to capture polymorphisms associated with antimalarial resistance and the genetic diversity within natural Plasmodium falciparum infections. Known genetic polymorphisms associated with altered drug susceptibility were screened for the five most common marker genes, pfdhfr, pfdhps, pfmdr1, pfcrt, and pfK13, and genetic diversity was established from two known AmpSeq markers, cpmp and csp. Relative abundance of the different genotypes within mixed infections was calculated from the number of reads per genotype. Genotyping was performed on 117 samples, 63 from asymptomatic and 54 from symptomatic individuals. We identified up to 15 genotypes within an infection, and the median multiplicity of infection was higher in asymptomatic infections (median MOI = 5 in asymptomatics versus median MOI = 2 in symptomatics, P < 0.001). No genetic differentiation on parasites from asymptomatic and symptomatic individuals was found. No mutation associated with ART resistance was identified. Prevalence of the P. falciparum chloroquine resistance wild-type genotype (CVMNK) reached 80%, confirming a return to chloroquine (CQ) sensitive parasites in Cameroon. In addition, the CQ-associated resistant genotype (CVIET) was present at very low density in polyclonal infections. Persistence of low-density chloroquine resistant parasites indicates competition-survival trade-offs may contribute to maintaining genetic diversity in natura. Thus, monitoring the expansion of these low-density genotypes in different immune backgrounds will be critical to evaluate drug policy changes. |
Hassan, A.; Blanchard, N. Microbial (co)infections: Powerful immune influencers Journal Article In: PLoS Pathog, vol. 18, no. 2, pp. e1010212, 2022, (doi: 10.1371/journal.ppat.1010212. eCollection 2022 Feb.). @article{c,It is well established that by modulating various immune functions, host infection may alter the course of concomitant inflammatory diseases, of both infectious and autoimmune etiologies. Beyond the major impact of commensal microbiota on the immune status, host exposure to viral, bacterial, and/or parasitic microorganisms also dramatically influences inflammatory diseases in the host, in a beneficial or harmful manner. Moreover, by modifying pathogen control and host tolerance to tissue damage, a coinfection can profoundly affect the development of a concomitant infectious disease. Here, we review the diverse mechanisms that underlie the impact of (co)infections on inflammatory disorders. We discuss epidemiological studies in the context of the hygiene hypothesis and shed light on the sometimes dual impact of germ exposure on human susceptibility to inflammatory disease. We then summarize the immunomodulatory mechanisms at play, which can involve pleiotropic effects of immune players and discuss the possibility to harness pathogen-derived compounds to the host benefit. |
2021 |
Charpentier, E.; Menard, S.; Marques, C.; Berry, A.; Iriart, X. Immune Response in Pneumocystis Infections According to the Host Immune System Status Journal Article In: J Fungi (Basel), vol. 7, no. 8, 2021, (doi: 10.3390/jof7080625.). @article{c,The host immune response is critical in Pneumocystis pneumonia (PCP). Immunocompetent hosts can eliminate the fungus without symptoms, while immunodeficient hosts develop PCP with an unsuitable excessive inflammatory response leading to lung damage. From studies based on rodent models or clinical studies, this review aimed to better understand the pathophysiology of Pneumocystis infection by analysing the role of immune cells, mostly lymphocytes, according to the immune status of the infected host. Hence, this review first describes the immune physiological response in infected immunocompetent hosts that are able to eliminate the fungus. The objective of the second part is to identify the immune elements required for the control of the fungus, focusing on specific immune deficiencies. Finally, the third part concentrates on the effect of the different immune elements in immunocompromised subjects during PCP, to better understand which cells are detrimental, and which, on the contrary, are beneficial once the disease has started. This work highlights that the immune response associated with a favourable outcome of the infection may differ according to the immune status of the host. In the case of immunocompetency, a close communication between B cells and TCD4 within tertiary lymphocyte structures appears critical to activate M2 macrophages without much inflammation. Conversely, in the case of immunodeficiency, a pro-inflammatory response including Th1 CD4, cytotoxic CD8, NK cells, and IFNgamma release seems beneficial for M1 macrophage activation, despite the impact of inflammation on lung tissue. |
Berry, A.; Menard, S.; Nsango, S. E.; Abate, L.; Concordet, D.; Tchioffo Tsapi, M.; Iriart, X.; Awono-Ambene, P. H.; Roche, B.; Morlais, I. The Rare, the Best: Spread of Antimalarial-Resistant Plasmodium falciparum Parasites by Anopheles Mosquito Vectors Journal Article In: Microbiol Spectr, vol. 9, no. 2, pp. e0085221, 2021, (doi: 10.1128/Spectrum.00852-21. Epub 2021 Oct 20.). @article{c,The emergence of resistance to antimalarials has prompted the steady switch to novel therapies for decades. Withdrawal of antimalarials, such as chloroquine in sub-Saharan Africa in the late 1990s, led to rapid declines in the prevalence of resistance markers after a few years, raising the possibility of reintroducing them for malaria treatment. Here, we provide evidence that the mosquito vector plays a crucial role in maintaining parasite genetic diversity. We followed the transmission dynamics of Plasmodium falciparum parasites through its vector in natural infections from gametocytes contained in the blood of asymptomatic volunteers until sporozoites subsequently developed in the mosquito salivary glands. We did not find any selection of the mutant or wild-type pfcrt 76 allele during development in the Anopheles mosquito vector. However, microsatellite genotyping indicated that minority genotypes were favored during transmission through the mosquito. The analysis of changes in the proportions of mutant and wild-type pfcrt 76 alleles showed that, regardless of the genotype, the less-represented allele in the gametocyte population was more abundant in mosquito salivary glands, indicating a selective advantage of the minority allele in the vector. Selection of minority genotypes in the vector would explain the persistence of drug-resistant alleles in the absence of drug pressure in areas with high malaria endemicity and high genetic diversity. Our results may have important epidemiological implications, as they predict the rapid re-emergence and spread of resistant genotypes if antimalarials that had previously selected resistant parasites are reintroduced for malaria prevention or treatment. IMPORTANCE Drug selection pressure in malaria patients is the cause of the emergence of resistant parasites. Resistance imposes a fitness cost for parasites in untreated infections, so withdrawal of the drug leads to the return of susceptible parasites. Little is known about the role of the malaria vector in this phenomenon. In an experimental study conducted in Cameroon, an area of high malaria transmission, we showed that the vector did not favor the parasites based on sensitivity or resistance criteria, but it did favor the selection of minority clones. This finding shows that the vector increases the diversity of plasmodial populations and could play an important role in falciparum malaria epidemiology by maintaining resistant clones despite the absence of therapeutic pressure. |
Charpentier, E.; Marques, C.; Menard, S.; Chauvin, P.; Guemas, E.; Cottrel, C.; Cassaing, S.; Fillaux, J.; Valentin, A.; Blanchard, N.; Berry, A.; Iriart, X. New Insights into Blood Circulating Lymphocytes in Human Pneumocystis Pneumonia Journal Article In: J Fungi (Basel), vol. 7, no. 8, 2021, (doi: 10.3390/jof7080652.). @article{c,The host lymphocyte response is decisive in Pneumocystis pneumonia (PCP) pathophysiology but little is known of the specific roles of lymphocyte subpopulations in this fungal infection. Peripheral NK, NKT, B, TCD4+ and TCD8+ subpopulations were compared by immunophenotyping between 20 patients diagnosed with PCP (PCP(+)] and 20 uninfected immunosuppressed patients (PCP(-)). Among PCP(+) subjects, the lymphocyte populations were also compared between surviving and deceased patients. Low B cell count (<40 cells/microL) was more frequent in PCP(+) than in PCP(-) patients (p = 0.03), while there was no difference for the TCD4 count. Among the PCP(+) group, the 7 deceased patients had lower Th1 (p = 0.02) and Tc1 (p = 0.03) populations, higher Th2 response (p = 0.03), higher effector TCD8 (p < 0.01), lower central memory TCD8 (p = 0.04) and reduced NK cells (p = 0.02) compared with the 13 survivors. Th1/Th2 ratio < 17, CD8 Tc1 < 44%, effector TCD8 < 25%, central memory TCD8 < 4%, NK cells < 50 cells/microL and total lymphocytes < 0.75 G/L were associated with a higher risk of mortality (p = 0.003 |
Huynh, J.; Baloyan, D.; Chisanga, D.; Shi, W.; O'Brien, M.; Afshar-Sterle, S.; Alorro, M.; Pang, L.; Williams, D. S.; Parslow, A. C.; Thilakasiri, P.; Eissmann, M. F.; Boon, L.; Masson, F.; Chand, A. L.; Ernst, M. Host IL11 Signaling Suppresses CD4(+) T cell-Mediated Antitumor Responses to Colon Cancer in Mice Journal Article In: Cancer Immunol Res, vol. 9, no. 7, pp. 735-747, 2021, (doi: 10.1158/2326-6066.CIR-19-1023. Epub 2021 Apr 27.). @article{c,IL11 is a member of the IL6 family of cytokines and signals through its cognate receptor subunits, IL11RA and glycoprotein 130 (GP130), to elicit biological responses via the JAK/STAT signaling pathway. IL11 contributes to cancer progression by promoting the survival and proliferation of cancer cells, but the potential immunomodulatory properties of IL11 signaling during tumor development have thus far remained unexplored. Here, we have characterized a role for IL11 in regulating CD4(+) T cell-mediated antitumor responses. Absence of IL11 signaling impaired tumor growth in a sporadic mouse model of colon cancer and syngeneic allograft models of colon cancer. Adoptive bone marrow transfer experiments and in vivo depletion studies demonstrated that the tumor-promoting activity of IL11 was mediated through its suppressive effect on host CD4(+) T cells in the tumor microenvironment. Indeed, when compared with Il11ra-proficient CD4(+) T cells associated with MC38 tumors, their Il11ra-deficient counterparts displayed elevated expression of mRNA encoding the antitumor mediators IFNgamma and TNFalpha. Likewise, IL11 potently suppressed the production of proinflammatory cytokines (IFNgamma, TNFalpha, IL6, and IL12p70) by CD4(+) T cells in vitro, which we corroborated by RNAscope analysis of human colorectal cancers, where IL11RA(high) tumors showed less IFNG and CD4 expression than IL11RA(low) tumors. Therefore, our results ascribe a tumor cell-extrinsic immunomodulatory role to IL11 during colon cancer development that could be amenable to an anticytokine-based therapy.See related Spotlight by van der Burg, p. 724. |
Mairet-Khedim, M.; Nsango, S.; Ngou, C.; Menard, S.; Roesch, C.; Khim, N.; Srun, S.; Iriart, X.; Lanot, T.; Otam, L.; Abega, F.; Ayong, L.; Morlais, I.; Gandia, P.; Witkowski, B.; Berry, A. Efficacy of dihydroartemisinin/piperaquine in patients with non-complicated Plasmodium falciparum malaria in Yaounde, Cameroon Journal Article In: J Antimicrob Chemother, vol. 76, no. 11, pp. 3037-3044, 2021, (doi: 10.1093/jac/dkab281.). @article{c,BACKGROUND: Dihydroartemisinin/piperaquine is increasingly used for the treatment of uncomplicated Plasmodium falciparum malaria in Africa. The efficacy of this combination in Cameroon is poorly documented, while resistance to dihydroartemisinin/piperaquine readily spreads in Southeast Asia. OBJECTIVES: This study evaluated the clinical efficacy of dihydroartemisinin/piperaquine in Cameroon, as well as the molecular profile and phenotypic susceptibility of collected isolates to dihydroartemisinin and piperaquine. PATIENTS AND METHODS: Dihydroartemisinin/piperaquine efficacy in 42 days was followed-up for 138 patients presenting non-complicated falciparum malaria. Piperaquine concentration was determined at day 7 for 124 patients. kelch13 gene polymorphisms (n = 150) and plasmepsin2 gene amplification (n = 148) were determined as molecular markers of resistance to dihydroartemisinin and piperaquine, respectively. Parasite susceptibility to dihydroartemisinin and piperaquine was determined using validated in vitro survival assays. RESULTS: The efficacy of dihydroartemisinin/piperaquine treatment was 100% after PCR correction. The reinfections were not associated with a variation of piperaquine concentration at day 7. Ninety-six percent (144/150) of the samples presented a WT allele of the kelch13 gene. Two percent (3/150) presented the non-synonymous mutation A578S, which is not associated with resistance to dihydroartemisinin. No duplication of the plasmepsin2 gene was observed (0/148). All the samples tested in vitro by survival assays (n = 87) were susceptible to dihydroartemisinin and piperaquine. CONCLUSIONS: Dihydroartemisinin/piperaquine has demonstrated excellent therapeutic efficacy with no evidence of emerging artemisinin or piperaquine resistance in Yaounde, Cameroon. This observation suggests that dihydroartemisinin/piperaquine could be a sustainable therapeutic solution for P. falciparum malaria if implemented in areas previously free of artemisinin- and piperaquine-resistant parasites, unlike Southeast Asia. |
Poncet, A. F.; Bosteels, V.; Hoffmann, E.; Chehade, S.; Rennen, S.; Huot, L.; Peucelle, V.; Marechal, S.; Khalife, J.; Blanchard, N.; Janssens, S.; Marion, S. The UPR sensor IRE1alpha promotes dendritic cell responses to control Toxoplasma gondii infection Journal Article In: EMBO Rep, vol. 22, no. 3, pp. e49617, 2021, (doi: 10.15252/embr.201949617. Epub 2021 Feb 15.). @article{c,The unfolded protein response (UPR) has emerged as a central regulator of immune cell responses in several pathologic contexts including infections. However, how intracellular residing pathogens modulate the UPR in dendritic cells (DCs) and thereby affect T cell-mediated immunity remains uncharacterized. Here, we demonstrate that infection of DCs with Toxoplasma gondii (T. gondii) triggers a unique UPR signature hallmarked by the MyD88-dependent activation of the IRE1alpha pathway and the inhibition of the ATF6 pathway. Induction of XBP1s controls pro-inflammatory cytokine secretion in infected DCs, while IRE1alpha promotes MHCI antigen presentation of secreted parasite antigens. In mice, infection leads to a specific activation of the IRE1alpha pathway, which is restricted to the cDC1 subset. Mice deficient for IRE1alpha and XBP1 in DCs display a severe susceptibility to T. gondii and succumb during the acute phase of the infection. This early mortality is correlated with increased parasite burden and a defect in splenic T-cell responses. Thus, we identify the IRE1alpha/XBP1s branch of the UPR as a key regulator of host defense upon T. gondii infection. |
Tuedom, A. G. B.; Sarah-Matio, E. M.; Moukoko, C. E. E.; Feufack-Donfack, B. L.; Maffo, C. N.; Bayibeki, A. N.; Awono-Ambene, H. P.; Ayong, L.; Berry, A.; Abate, L.; Morlais, I.; Nsango, S. E. Antimalarial drug resistance in the Central and Adamawa regions of Cameroon: Prevalence of mutations in P. falciparum crt, Pfmdr1, Pfdhfr and Pfdhps genes Journal Article In: PLoS One, vol. 16, no. 8, pp. e0256343, 2021, (doi: 10.1371/journal.pone.0256343. eCollection 2021.). @article{c,The spread of Plasmodium falciparum resistant parasites remains one of the major challenges for malaria control and elimination in Sub Saharan Africa. Monitoring of molecular markers conferring resistance to different antimalarials is important to track the spread of resistant parasites and to optimize the therapeutic lifespan of current drugs. This study aimed to evaluate the prevalence of known mutations in the drug resistance genes Pfcrt, Pfmdr1, Pfdhfr and Pfdhps in two different epidemiological settings in Cameroon. Dried blood spots collected in 2018 and 2019 from asymptomatic individuals were used for DNA extraction and then the Plasmodium infection status was determined byPCR. Detection of SNPs was performed by nested PCR followed by allele-specific restriction analysis (ASRA). The prevalence of each genotype was compared between sites using the Chi square and Fisher's exact tests. A high prevalence of the Pfcrt K76 wild type allele was found in both sites (88.5 and 62.29% respectively; P< 0,0001). The prevalence of Pfmdr1 mutations 86Y and 1246Y was respectively 55.83 and 1.45% in Mfou and 45.87 and 5.97% in Tibati, with significant difference between the studied areas (P<0.0001). Overall, the Pfdhfr triple-mutant genotype (51I/59R/108N) was highly prevalent (> 96%), however no SNP was detected at codon 164. In Pfdhps, the prevalence of the 437G mutation reached (90%) and was at higher frequency in Mfou (P< 0.0001). Overall, the Pfdhps mutations 540E and 581G were less common (0.33 and 3.26%, respectively). The quadruple resistant genotype (Pfdhfr 51I/59R/108N+Pfdhp437G) was found almost 90% of the samples. The wild-type genotype (Pfdhfr N51/C59/S108/164I+Pfdhps A437/K540/A581) was never identified and the sextuple mutant (Pfdhfr 51I/59R/108N+Pfdhp437G/540E/581G), kwon as super resistant appeared in two samples from Tibati. These findings demonstrate declining trends in the prevalence of mutations conferring resistance to 4-aminoquinolines, especially to chloroquine. However, a high level of mutations in P. falciparum genes related to SP resistance was detected and this raises concerns about the future efficacy of IPTp-SP and SMC in Cameroon. |
2020 |
Laing, C.; Blanchard, N.; McConkey, G. A. Noradrenergic Signaling and Neuroinflammation Crosstalk Regulate Toxoplasma gondii-Induced Behavioral Changes Journal Article In: Trends Immunol, vol. 41, no. 12, pp. 1072-1082, 2020, (doi: 10.1016/j.it.2020.10.001. Epub 2020 Nov 16.). @article{c,Infections of the nervous system elicit neuroimmune responses and alter neurotransmission, affecting host neurological functions. Chronic infection with the apicomplexan parasite Toxoplasma correlates with certain neurological disorders in humans and alters behavior in rodents. Here, we propose that the crosstalk between neurotransmission and neuroinflammation may underlie some of these cognitive changes. We discuss how T. gondii infection suppresses noradrenergic signaling and how the restoration of this pathway improves behavioral aberrations, suggesting that altered neurotransmission and neuroimmune responses may act in concert to perturb behavior. This interaction might apply to other infectious agents, such as viruses, that elicit cognitive changes. We hypothesize that neurotransmitter signaling in immune cells can contribute to behavioral changes associated with brain infection, offering opportunities for potential therapeutic targeting. |
Kaminski, H.; Belliere, J.; Burguet, L.; Del Bello, A.; Taton, B.; Poirot-Mazeres, S.; Accoceberry, I.; Delhaes, L.; Visentin, J.; Gregori, M.; Iriart, X.; Charpentier, E.; Couzi, L.; Kamar, N.; Merville, P. Identification of predictive markers and outcomes of late-onset Pneumocystis jirovecii pneumonia in kidney transplant recipients Journal Article In: Clin Infect Dis, 2020, (doi: 10.1093/cid/ciaa1611.). @article{c,BACKGROUND: In the era of prophylaxis, Pneumocystis pneumonia has become a late-onset opportunistic infection requiring indications for prolonged prophylaxis to be defined. The primary objective of our study was therefore to evaluate risk factors associated with late-onset Pneumocystis pneumonia. The secondary objective was to assess the impact of this infection on graft and patient survival. METHODS: We conducted a French case-control study in Bordeaux and Toulouse center by matching 1 case to 1 or 2 controls from the same center based on the transplant date and the type of induction treatment. RESULTS: Seventy cases and 134 controls were included. Pneumocystis pneumonia occurred at a median of 3 years after transplantation. The total lymphocyte count, CD4+ and CD8+ T lymphocytes values were lower in the cases than in their matched controls on the day of infection and annually up to 4 years earlier. The covariables independently associated with Pneumocystis pneumonia were the total lymphocyte count one year before Pneumocystis, mTOR inhibitors used as maintenance immunosuppressive drugs and the administration of corticosteroid boluses used in acute rejection. A total lymphocyte count threshold <1000/microL offered the best predictive value for infection occurrence. Pneumocystis pneumonia was associated with high incidence of graft loss and patient death (30% and 17% respectively, three years after PCP). CONCLUSIONS: Pneumocystis pneumonia has dramatic consequences in kidney transplant recipients and a targeted prophylaxis based on simple criteria, such as chronic lymphopenia and/or history of corticosteroid boluses could be useful to avoid life-threatening complications. |
Iriart, X.; Menard, S.; Chauvin, P.; Mohamed, H. S.; Charpentier, E.; Mohamed, M. A.; Berry, A.; Aboubaker, M. H. Misdiagnosis of imported falciparum malaria from African areas due to an increased prevalence of pfhrp2/pfhrp3 gene deletion: the Djibouti case Journal Article In: Emerg Microbes Infect, vol. 9, no. 1, pp. 1984-1987, 2020, (doi: 10.1080/22221751.2020.1815590.). @article{c,Following the diagnosis of a falciparum malaria case imported from Djibouti and not detected by a pfHRP2-based rapid diagnostic test (RDT), we investigated the prevalence of the pfhrp2/pfhrp3-deleted parasites in Djibouti using 378 blood samples collected between January and May 2019, from Djiboutian patients with suspected malaria. Malaria diagnosis by quantitative PCR confirmed the presence of Plasmodium falciparum for 20.9% (79/378) samples while RDTs did not detect HRP2 antigen in 83.5% (66/79) of these samples. Quantitative PCRs targeting the pfhrp2/pfhrp3 genes confirmed the absence of both genes for 86.5% of P. falciparum strains. The very large number (86.5%) of falciparum parasites lacking the pfhrp2/pfhrp3 genes observed in this study, now justifies the use of non-HRP2 alternative RDTs in Djibouti. In this area and in most countries where HRP2-based RDTs constitute the main arsenal for falciparum malaria diagnosis, it is important to implement a systematic surveillance and to inform biologists and clinicians about the risk of malaria misdiagnosis. Further investigations are needed to better understand the mechanism of selection and diffusion of the pfhrp2/pfhrp3-deleted parasites. |
Hassan, A.; Wlodarczyk, M. F.; Benamar, M.; Bassot, E.; Salvioni, A.; Kassem, S.; Berry, A.; Saoudi, A.; Blanchard, N. A Virus Hosted in Malaria-Infected Blood Protects against T Cell-Mediated Inflammatory Diseases by Impairing DC Function in a Type I IFN-Dependent Manner Journal Article In: mBio, vol. 11, no. 2, 2020, (doi: 10.1128/mBio.03394-19.). @article{c,Coinfections shape immunity and influence the development of inflammatory diseases, resulting in detrimental or beneficial outcome. Coinfections with concurrent Plasmodium species can alter malaria clinical evolution, and malaria infection itself can modulate autoimmune reactions. Yet, the underlying mechanisms remain ill defined. Here, we demonstrate that the protective effects of some rodent malaria strains on T cell-mediated inflammatory pathologies are due to an RNA virus cohosted in malaria-parasitized blood. We show that live and extracts of blood parasitized by Plasmodium berghei K173 or Plasmodium yoelii 17X YM, protect against P. berghei ANKA-induced experimental cerebral malaria (ECM) and myelin oligodendrocyte glycoprotein (MOG)/complete Freund's adjuvant (CFA)-induced experimental autoimmune encephalomyelitis (EAE), and that protection is associated with a strong type I interferon (IFN-I) signature. We detected the presence of the RNA virus lactate dehydrogenase-elevating virus (LDV) in the protective Plasmodium stabilates and we established that LDV infection alone was necessary and sufficient to recapitulate the protective effects on ECM and EAE. In ECM, protection resulted from an IFN-I-mediated reduction in the abundance of splenic conventional dendritic cell and impairment of their ability to produce interleukin (IL)-12p70, leading to a decrease in pathogenic CD4(+) Th1 responses. In EAE, LDV infection induced IFN-I-mediated abrogation of IL-23, thereby preventing the differentiation of granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing encephalitogenic CD4(+) T cells. Our work identifies a virus cohosted in several Plasmodium stabilates across the community and deciphers its major consequences on the host immune system. More generally, our data emphasize the importance of considering contemporaneous infections for the understanding of malaria-associated and autoimmune diseases.IMPORTANCE Any infection modifies the host immune status, potentially ameliorating or aggravating the pathophysiology of a simultaneous inflammatory condition. In the course of investigating how malaria infection modulates the severity of contemporaneous inflammatory diseases, we identified a nonpathogenic mouse virus in stabilates of two widely used rodent parasite lines: Plasmodium berghei K173 and Plasmodium yoelii 17X YM. We established that the protective effects of these Plasmodium lines on cerebral malaria and multiple sclerosis are exclusively due to this virus. The virus induces a massive type I interferon (IFN-I) response and causes quantitative and qualitative defects in the ability of dendritic cells to promote pathogenic T cell responses. Beyond revealing a possible confounding factor in rodent malaria models, our work uncovers some bases by which a seemingly innocuous viral (co)infection profoundly changes the immunopathophysiology of inflammatory diseases. |
Charpentier, E.; Benichou, E.; Pages, A.; Chauvin, P.; Fillaux, J.; Valentin, A.; Guegan, H.; Guemas, E.; Salabert, A. S.; Armengol, C.; Menard, S.; Cassaing, S.; Berry, A.; Iriart, X. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria Journal Article In: Clin Microbiol Infect, vol. 26, no. 1, pp. 115-121, 2020, (doi: 10.1016/j.cmi.2019.05.010. Epub 2019 May 31.). @article{c,OBJECTIVES: Malaria is one of most common tropical diseases encountered in travellers and migrants. It requires an urgent and reliable diagnosis considering its potential severity. In this study, performance of five diagnostic assays were evaluated in a nonendemic region and compared prospectively to quantitative PCR (qPCR). METHODS: A prospective study was conducted at Toulouse Hospital from August 2017 to January 2018 and included all patients with initial Plasmodium screening. Thin and thick blood smears (TnS, TkS), quantitative buffy coat (QBC), rapid diagnostic tests (RDTs) and commercial loop-mediated isothermal amplification (LAMP) were independently performed on each blood sample and compared to our qPCR reference standard. RESULTS: The study encompassed 331 patients, mainly returning from Africa. qPCR detected 73 Plasmodium-positive samples (including 58 falciparum). Individually, LAMP had a 97.3% (71/73) sensitivity, far ahead of TnS (84.9%, 62/73), TkS (86.3%, 63/73), QBC (86.3%, 63/73) and RDT (86.3%, 63/73). RDT demonstrated a high sensitivity for falciparum (98.3%, 57/58) but missed all ovale, malariae and knowlesi infections. Specificity was excellent for all techniques (99.6-100%). The most sensitive diagnosis strategies were TnS + RDT (95.9%, 70/73), TnS + LAMP (97.3%, 71/73) and TnS + RDT + LAMP (100%, 73/73), about 10% higher than strategies using exclusively microscopy, TkS + TnS (87.7%, 64/73) or QBC + TnS (87.7%, 64/73). TnS remains necessary for Plasmodium species identification and quantification. Adding sequentially TnS only on LAMP-positive samples did not decrease TnS + LAMP strategy sensitivity. CONCLUSIONS: In nonendemic countries, the currently recommended microscopy-based strategies seem unsatisfactory for malaria diagnosis considering RDT and LAMP performance, two rapid and sensitive assays that require limited training. |
Cerutti, A.; Blanchard, N.; Besteiro, S. The Bradyzoite: A Key Developmental Stage for the Persistence and Pathogenesis of Toxoplasmosis Journal Article In: Pathogens, vol. 9, no. 3, 2020, (doi: 10.3390/pathogens9030234.). @article{c,Toxoplasma gondii is a ubiquitous parasitic protist found in a wide variety of hosts, including a large proportion of the human population. Beyond an acute phase which is generally self-limited in immunocompetent individuals, the ability of the parasite to persist as a dormant stage, called bradyzoite, is an important aspect of toxoplasmosis. Not only is this stage not eliminated by current treatments, but it can also reactivate in immunocompromised hosts, leading to a potentially fatal outcome. Yet, despite its critical role in the pathology, the bradyzoite stage is relatively understudied. One main explanation is that it is a considerably challenging model, which essentially has to be derived from in vivo sources. However, recent progress on genetic manipulation and in vitro differentiation models now offers interesting perspectives for tackling key biological questions related to this particularly important developmental stage. |
2019 |
Guegan, H.; Fillaux, J.; Charpentier, E.; Robert-Gangneux, F.; Chauvin, P.; Guemas, E.; Boissier, J.; Valentin, A.; Cassaing, S.; Gangneux, J. P.; Berry, A.; Iriart, X. Real-time PCR for diagnosis of imported schistosomiasis Journal Article In: PLoS Negl Trop Dis, vol. 13, no. 9, pp. e0007711, 2019. @article{b,BACKGROUND: The diagnosis of schistosomiasis currently relies on microscopic detection of schistosome eggs in stool or urine samples and serological assays. The poor sensitivity of standard microscopic procedures performed in routine laboratories, makes molecular detection methods of increasing interest. The aim of the study was to evaluate two in-house real-time Schistosoma PCRs, targeting respectively S. mansoni [Sm] and S. haematobium [Sh] in excreta, biopsies and sera as potential tools to diagnose active infections and to monitor treatment efficacy. METHODS: Schistosoma PCRs were performed on 412 samples (124 urine, 86 stools, 8 biopsies, 194 sera) from patients with suspected schistosomiasis, before anti-parasitic treatment. Results were compared to microscopic examination and serological assays (enzyme-linked immunosorbent assay (ELISA), indirect haemagglutination (HA) and Western Blot (WB) assay). RESULTS: Compared to microscopy, PCRs significantly increased the sensitivity of diagnosis, from 4% to 10.5% and from 33.7% to 48.8%, for Sh in urine and Sm in stools, respectively. The overall sensitivity of PCR on serum samples was 72.7% and reached 94.1% in patients with positive excreta (microscopy). The specificity of serum PCR was 98.9%. After treatment, serum PCR positivity rates slowly declined from 93.8% at day 30 to 8.3% at day 360, whereas antibody detection remained positive after 1 year. CONCLUSION: Schistosoma PCRs clearly outperform standard microscopy on stools and urine and could be part of reference methods combined with WB-based serology, which remains a gold standard for initial diagnosis. When serological assays are positive and microscopy is negative, serum PCRs provide species information to guide further clinical exploration. Biomarkers such as DNA and antibodies are of limited relevance for early treatment monitoring but serum PCR could be useful when performed at least 1 year after treatment to help confirm a cured infection. |
Salvioni, A.; Belloy, M.; Lebourg, A.; Bassot, E.; Cantaloube-Ferrieu, V.; Vasseur, V.; Blanie, S.; Liblau, R. S.; Suberbielle, E.; Robey, E. A.; Blanchard, N. Robust Control of a Brain-Persisting Parasite through MHC I Presentation by Infected Neurons Journal Article In: Cell Rep, vol. 27, no. 11, pp. 3254-3268 e8, 2019, (Jun 11;27(11):3254-3268.e8. doi: 10.1016/j.celrep.2019.05.051.). @article{b,Control of CNS pathogens by CD8 T cells is key to avoid fatal neuroinflammation. Yet, the modalities of MHC I presentation in the brain are poorly understood. Here, we analyze the antigen presentation mechanisms underlying CD8 T cell-mediated control of the Toxoplasma gondii parasite in the CNS. We show that MHC I presentation of an efficiently processed model antigen (GRA6-OVA), even when not expressed in the bradyzoite stage, reduces cyst burden and dampens encephalitis in C57BL/6 mice. Antigen presentation assays with infected primary neurons reveal a correlation between lower MHC I presentation of tachyzoite antigens by neurons and poor parasite control in vivo. Using conditional MHC I-deficient mice, we find that neuronal MHC I presentation is required for robust restriction of T. gondii in the CNS during chronic phase, showing the importance of MHC I presentation by CNS neurons in the control of a prevalent brain pathogen. |
Poncet, A. F.; Blanchard, N.; Marion, S. Toxoplasma and Dendritic Cells: An Intimate Relationship That Deserves Further Scrutiny Journal Article In: Trends Parasitol, 2019, (Sep 3. pii: S1471-4922(19)30210-7. doi: 10.1016/j.pt.2019.08.001.). @article{b,Toxoplasma gondii (Tg), an obligate intracellular parasite of the phylum Apicomplexa, infects a wide range of animals, including humans. A hallmark of Tg infection is the subversion of host responses, which is thought to favor parasite persistence and propagation to new hosts. Recently, a variety of parasite-secreted modulatory effectors have been uncovered in fibroblasts and macrophages, but the specific interplay between Tg and dendritic cells (DCs) is just beginning to emerge. In this review, we summarize the current knowledge on Tg-DC interactions, including innate recognition, cytokine production, and antigen presentation, and discuss open questions regarding how Tg-secreted effectors may shape DC functions to perturb innate and adaptive immunity. |
Charpentier, E.; Benichou, E.; Pages, A.; Chauvin, P.; Fillaux, J.; Valentin, A.; Guegan, H.; Guemas, E.; Salabert, A. S.; Armengol, C.; Menard, S.; Cassaing, S.; Berry, A.; Iriart, X. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria Journal Article In: Clin Microbiol Infect, 2019, (May 31. pii: S1198-743X(19)30225-3. doi: 10.1016/j.cmi.2019.05.010.). @article{b,OBJECTIVES: Malaria is one of most common tropical diseases encountered in travellers and migrants. It requires an urgent and reliable diagnosis considering its potential severity. In this study, performance of five diagnostic assays were evaluated in a nonendemic region and compared prospectively to quantitative PCR (qPCR). METHODS: A prospective study was conducted at Toulouse Hospital from August 2017 to January 2018 and included all patients with initial Plasmodium screening. Thin and thick blood smears (TnS, TkS), quantitative buffy coat (QBC), rapid diagnostic tests (RDTs) and commercial loop-mediated isothermal amplification (LAMP) were independently performed on each blood sample and compared to our qPCR reference standard. RESULTS: The study encompassed 331 patients, mainly returning from Africa. qPCR detected 73 Plasmodium-positive samples (including 58 falciparum). Individually, LAMP had a 97.3% (71/73) sensitivity, far ahead of TnS (84.9%, 62/73), TkS (86.3%, 63/73), QBC (86.3%, 63/73) and RDT (86.3%, 63/73). RDT demonstrated a high sensitivity for falciparum (98.3%, 57/58) but missed all ovale, malariae and knowlesi infections. Specificity was excellent for all techniques (99.6-100%). The most sensitive diagnosis strategies were TnS + RDT (95.9%, 70/73), TnS + LAMP (97.3%, 71/73) and TnS + RDT + LAMP (100%, 73/73), about 10% higher than strategies using exclusively microscopy, TkS + TnS (87.7%, 64/73) or QBC + TnS (87.7%, 64/73). TnS remains necessary for Plasmodium species identification and quantification. Adding sequentially TnS only on LAMP-positive samples did not decrease TnS + LAMP strategy sensitivity. CONCLUSIONS: In nonendemic countries, the currently recommended microscopy-based strategies seem unsatisfactory for malaria diagnosis considering RDT and LAMP performance, two rapid and sensitive assays that require limited training. |
2018 |
Niare, K.; Paloque, L.; Menard, S.; Tor, P.; Ramadani, A. P.; Augereau, J. M.; Dara, A.; Berry, A.; Benoit-Vical, F.; Doumbo, O. K. Multiple Phenotypic and Genotypic Artemisinin Sensitivity Evaluation of Malian Plasmodium falciparum Isolates Journal Article In: Am J Trop Med Hyg, 2018, (Feb 12. doi: 10.4269/ajtmh.17-0798.). @article{b,We assessed the ex vivo/in vitro sensitivity of 54 Malian Plasmodium falciparum isolates to artemisinin for the monitoring of drug resistance in this area. The artemisinin sensitivity of parasites was evaluated using 1) the ex vivo and in vitro parasite recrudescence detection after treatment of the ring stage with 1-200 nM artemisinin for 48 hours and 2) the in vitro parasite recrudescence kinetics assay over 7 days after 6-hour treatment of the ring stage with 700 nM dihydroartemisinin (DHA). In addition, as recommended by the World Health Organization for artemisinin resistance characterization, the ring-stage survival assay (RSA(0-3 h)) was performed and the parasite isolates were sequenced at the kelch 13 propeller locus. No clinical and molecular evidence of artemisinin resistance was observed. However, these isolates present different phenotypic profiles in response to artemisinin treatments. Despite all RSA(0-3 h) values less than 1.5%, six out of 46 (13.0%) isolates tested ex vivo and four out of six (66.7%) isolates tested in vitro were able to multiply after 48-hour treatments with 100 nM artemisinin. Moreover, five out of eight isolates tested showed faster parasite recovery after DHA treatment in kinetic assays. The presence of such phenotypes needs to be taken into account in the assessment of the efficacy of artemisinins in Mali. The assays presented here appear as valuable tools for the monitoring of artemisinin sensitivity in the field and thus could help to evaluate the risk of emergence of artemisinin resistance in Africa. |
2017 |
Santi-Rocca, J.; Blanchard, N. Membrane trafficking and remodeling at the host-parasite interface Journal Article In: Curr Opin Microbiol, vol. 40, pp. 145-151, 2017, (Dec;40:145-151. doi: 10.1016/j.mib.2017.11.013. Epub 2017 Nov 22.). @article{b,Membrane shape is functionally linked with many cellular processes. The limiting membrane of vacuoles containing Toxoplasma gondii and Plasmodium apicomplexan parasites lies at the host-parasite interface. This membrane comprises intra-vacuolar and extra-vacuolar tubulo-vesicular deformations, which influence host-parasite cross-talk. Here, underscoring specificities and similarities between the T. gondii and Plasmodium contexts, we present recent findings about vacuolar membrane remodeling and its potential roles in parasite fitness and immune recognition. We review in particular the implication of tubulo-vesicular structures in trapping and/or transporting host and parasite components. Understanding how membrane remodeling influences host-pathogen interactions is expected to be critical in the battle against many intracellular pathogens beyond parasites. |
Bonnart, C.; Feuillet, G.; Vasseur, V.; Cenac, N.; Vergnolle, N.; Blanchard, N. Protease-Activated Receptor 2 contributes to Toxoplasma gondii-mediated gut inflammation Journal Article In: Parasite Immunol, 2017, (Sep 7. doi: 10.1111/pim.12489.). @article{b,Toxoplasma gondii is a widespread intracellular parasite, which naturally enters the organism via the oral route and cross the intestinal barrier to disseminate. In addition to neuronal and ocular pathologies, this pathogen also causes gut inflammation in a number of animals. This infection-triggered inflammation has been extensively studied in the C57BL/6 mice, highlighting the importance of the immune cells and their mediators in the development of gut pathology. However, despite their importance in inflammation, the role of Protease-Activated Receptors (PAR) was never reported in the context of T.gondii-mediated small intestine inflammation. Using genetically modified mice, we show that PAR2 plays a pathogenic role in the development of gut inflammatory lesions. We find that PAR2 controls the innate inflammatory mediators IL-6, KC/CXCL1, PGE2 as well as neutrophil infiltration in T. gondii-triggered gut damage. These results bring new knowledge on the mechanisms operating in the gut in response to T. gondii infection. This article is protected by copyright. All rights reserved. |
Buaillon, C.; Guerrero, N. A.; Cebrian, I.; Blanie, S.; Lopez, J.; Bassot, E.; Vasseur, V.; Santi-Rocca, J.; Blanchard, N. MHC I presentation of Toxoplasma gondii immunodominant antigen does not require Sec22b and is regulated by antigen orientation at the vacuole membrane Journal Article In: Eur J Immunol, vol. 47, no. 7, pp. 1160-1170, 2017, (Jul;47(7):1160-1170. doi: 10.1002/eji.201646859. Epub 2017 Jun 8.). @article{b,The intracellular Toxoplasma gondii parasite replicates within a parasitophorous vacuole (PV). T. gondii secretes proteins that remain soluble in the PV space, are inserted into PV membranes or are exported beyond the PV boundary. In addition to supporting T. gondii growth, these proteins can be processed and presented by MHC I for CD8+ T-cell recognition. Yet it is unclear whether membrane binding influences the processing pathways employed and if topology of membrane antigens impacts their MHC I presentation. Here we report that the MHC I pathways of soluble and membrane-bound antigens differ in their requirement for host ER recruitment. In contrast to the soluble SAG1-OVA model antigen, we find that presentation of the membrane-bound GRA6 is independent from the SNARE Sec22b, a key molecule for transfer of host endoplasmic reticulum components onto the PV. Using parasites modified to secrete a transmembrane antigen with opposite orientations, we further show that MHC I presentation is highly favored when the C-terminal epitope is exposed to the host cell cytosol, which corresponds to GRA6 natural orientation. Our data suggest that the biochemical properties of antigens released by intracellular pathogens critically guide their processing pathway and are valuable parameters to consider for vaccination strategies. |
Draheim, M.; Wlodarczyk, M. F.; Crozat, K.; Saliou, J. M.; Alayi, T. D.; Tomavo, S.; Hassan, A.; Salvioni, A.; Demarta-Gatsi, C.; Sidney, J.; Sette, A.; Dalod, M.; Berry, A.; Silvie, O.; Blanchard, N. Profiling MHC II immunopeptidome of blood-stage malaria reveals that cDC1 control the functionality of parasite-specific CD4 T cells Journal Article In: EMBO Mol Med, 2017, (Sep 21. pii: e201708123. doi: 10.15252/emmm.201708123.). @article{b,In malaria, CD4 Th1 and T follicular helper (TFH) cells are important for controlling parasite growth, but Th1 cells also contribute to immunopathology. Moreover, various regulatory CD4 T-cell subsets are critical to hamper pathology. Yet the antigen-presenting cells controlling Th functionality, as well as the antigens recognized by CD4 T cells, are largely unknown. Here, we characterize the MHC II immunopeptidome presented by DC during blood-stage malaria in mice. We establish the immunodominance hierarchy of 14 MHC II ligands derived from conserved parasite proteins. Immunodominance is shaped differently whether blood stage is preceded or not by liver stage, but the same ETRAMP-specific dominant response develops in both contexts. In naive mice and at the onset of cerebral malaria, CD8alpha+ dendritic cells (cDC1) are superior to other DC subsets for MHC II presentation of the ETRAMP epitope. Using in vivo depletion of cDC1, we show that cDC1 promote parasite-specific Th1 cells and inhibit the development of IL-10+ CD4 T cells. This work profiles the P. berghei blood-stage MHC II immunopeptidome, highlights the potency of cDC1 to present malaria antigens on MHC II, and reveals a major role for cDC1 in regulating malaria-specific CD4 T-cell responses. |
Lebrun, M.; Blanchard, N. Editorial overview: Host-microbe interactions: parasites Journal Article In: Curr Opin Microbiol, vol. 40, pp. viii-xi, 2017, (Dec;40:viii-xi. doi: 10.1016/j.mib.2017.11.028.). @article{b, |
Keita Alassane, S.; Nicolau-Travers, M. L.; Menard, S.; Andreoletti, O.; Cambus, J. P.; Gaudre, N.; Wlodarczyk, M.; Blanchard, N.; Berry, A.; Abbes, S.; Colongo, D.; Faye, B.; Augereau, J. M.; Lacroux, C.; Iriart, X.; Benoit-Vical, F. Young Sprague Dawley rats infected by Plasmodium berghei: A relevant experimental model to study cerebral malaria Journal Article In: PLoS One, vol. 12, no. 7, pp. e0181300, 2017, (Jul 24;12(7):e0181300. doi: 10.1371/journal.pone.0181300. eCollection 2017.). @article{b,Cerebral malaria (CM) is the most severe manifestation of human malaria yet is still poorly understood. Mouse models have been developed to address the subject. However, their relevance to mimic human pathogenesis is largely debated. Here we study an alternative cerebral malaria model with an experimental Plasmodium berghei Keyberg 173 (K173) infection in Sprague Dawley rats. As in Human, not all infected subjects showed cerebral malaria, with 45% of the rats exhibiting Experimental Cerebral Malaria (ECM) symptoms while the majority (55%) of the remaining rats developed severe anemia and hyperparasitemia (NoECM). These results allow, within the same population, a comparison of the noxious effects of the infection between ECM and severe malaria without ECM. Among the ECM rats, 77.8% died between day 5 and day 12 post-infection, while the remaining rats were spontaneously cured of neurological signs within 24-48 hours. The clinical ECM signs observed were paresis quickly evolving to limb paralysis, global paralysis associated with respiratory distress, and coma. The red blood cell (RBC) count remained normal but a drastic decrease of platelet count and an increase of white blood cell numbers were noted. ECM rats also showed a decrease of glucose and total CO2 levels and an increase of creatinine levels compared to control rats or rats with no ECM. Assessment of the blood-brain barrier revealed loss of integrity, and interestingly histopathological analysis highlighted cyto-adherence and sequestration of infected RBCs in brain vessels from ECM rats only. Overall, this ECM rat model showed numerous clinical and histopathological features similar to Human CM and appears to be a promising model to achieve further understanding the CM pathophysiology in Humans and to evaluate the activity of specific antimalarial drugs in avoiding/limiting cerebral damages from malaria. |
2016 |
Tchioffo, M. T.; Abate, L.; Boissiere, A.; Nsango, S. E.; Gimonneau, G.; Berry, A.; Oswald, E.; Dubois, D.; Morlais, I. An epidemiologically successful Escherichia coli sequence type modulates Plasmodium falciparum infection in the mosquito midgut Journal Article In: Infect Genet Evol, vol. 43, pp. 22-30, 2016, (Sep;43:22-30. doi: 10.1016/j.meegid.2016.05.002. Epub 2016 May 3.). @article{b,Malaria transmission relies on the successful development of Plasmodium parasites in the Anopheles mosquito vector. Within the mosquito midgut, malaria parasites encounter a resident bacterial flora and parasite-bacteria interactions modulate Plasmodium development. The mechanisms by which the bacteria interact with malaria parasites are still unknown. The intestinal microbiota could regulate immune signaling pathways or produce bacterial compounds that block Plasmodium development. In this study, we characterized Escherichia coli strains previously isolated from the Anopheles mosquito midgut and investigated the putative role of two E. coli clones, 444ST95 and 351ST73, on parasite development. Sporogonic development was significantly impacted by exposure to clone 444ST95 whereas prevalence and intensity of infection were not different in mosquitoes challenged with 351ST73 as compared to control mosquitoes. This result indicates midgut bacteria exhibit intra-specific variation in their ability to inhibit Plasmodium development. Expression patterns of immune genes differed between mosquitoes challenged with 444ST95 and 351ST73 and examination of the luminal midgut surface by transmission electron microscopy revealed distinct effects of bacterial exposure on midgut epithelial cells. The 444ST95 clone strongly affected mosquito survival and parasite development and this could be associated to the Hemolysin F or other toxins released by the bacteria. Further studies will be needed to decipher the virulence factors and to determine their contribution to the observed phenotype of the 444ST95E. coli strain that belongs to the epidemiological ST95 clonal group responsible for extra intestinal infections in human and other animals. |
Cebrian, I.; Croce, C.; Guerrero, N. A.; Blanchard, N.; Mayorga, L. S. Rab22a controls MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells Journal Article In: EMBO Rep, 2016, (Oct 10. pii: e201642358.). @article{b,Cross-presentation by MHC class I molecules allows the detection of exogenous antigens by CD8+ T lymphocytes. This process is crucial to initiate cytotoxic immune responses against many pathogens (i.e., Toxoplasma gondii) and tumors. To achieve efficient cross-presentation, dendritic cells (DCs) have specialized endocytic pathways; however, the molecular effectors involved are poorly understood. In this work, we identify the small GTPase Rab22a as a key regulator of MHC-I trafficking and antigen cross-presentation by DCs. Our results demonstrate that Rab22a is recruited to DC endosomes and phagosomes, as well as to the vacuole containing T. gondii parasites. The silencing of Rab22a expression did not affect the uptake of exogenous antigens or parasite invasion, but it drastically reduced the intracellular pool and the recycling of MHC-I molecules. The knockdown of Rab22a also hampered the cross-presentation of soluble, particulate and T. gondii-associated antigens, but not the endogenous MHC-I antigen presentation through the classical secretory pathway. Our findings provide compelling evidence that Rab22a plays a central role in the MHC-I endocytic trafficking, which is crucial for efficient cross-presentation by DCs. |
Chu, H. H.; Chan, S. W.; Gosling, J. P.; Blanchard, N.; Tsitsiklis, A.; Lythe, G.; Shastri, N.; Molina-Paris, C.; Robey, E. A. Continuous Effector CD8(+) T Cell Production in a Controlled Persistent Infection Is Sustained by a Proliferative Intermediate Population Journal Article In: Immunity, vol. 45, no. 1, pp. 159-71, 2016, (Jul 19;45(1):159-71. doi: 10.1016/j.immuni.2016.06.013. Epub 2016 Jul 12.). @article{b,Highly functional CD8(+) effector T (Teff) cells can persist in large numbers during controlled persistent infections, as exemplified by rare HIV-infected individuals who control the virus. Here we examined the cellular mechanisms that maintain ongoing T effector responses using a mouse model for persistent Toxoplasma gondii infection. In mice expressing the protective MHC-I molecule, H-2L(d), a dominant T effector response against a single parasite antigen was maintained without a contraction phase, correlating with ongoing presentation of the dominant antigen. Large numbers of short-lived Teff cells were continuously produced via a proliferative, antigen-dependent intermediate (Tint) population with a memory-effector hybrid phenotype. During an acute, resolved infection, decreasing antigen load correlated with a sharp drop in the Tint cell population and subsequent loss of the ongoing effector response. Vaccination approaches aimed at the development of Tint populations might prove effective against pathogens that lead to chronic infection. |
Latour, C.; Wlodarczyk, M. F.; Jung, G.; Gineste, A.; Blanchard, N.; Ganz, T.; Roth, M. P.; Coppin, H.; Kautz, L. Erythroferrone contributes to hepcidin repression in a mouse model of malarial anemia Journal Article In: Haematologica, 2016, (Sep 22. pii: haematol.2016.150227.). @article{b,Malaria, a major global health challenge worldwide, is accompanied by a severe anemia secondary to hemolysis and increased erythrophagocytosis. Iron is an essential functional component of erythrocyte hemoglobin and its availability is controlled by the liver-derived hormone hepcidin. We examined the regulation of hepcidin during malarial infection in mice using the rodent parasite Plasmodium berghei K173 (PbK). Mice infected with PbK develop a severe anemia and die after 18 to 22 days without cerebral malaria. During the early phase of blood-stage infection (day 1 to 5), a strong inflammatory signature was associated with increased production of hepcidin. Between days 7 and 18, while infection progresses, red blood cell count, hemoglobin and hematocrit dramatically decreased. In the late phase of malarial infection, hepcidin production was reduced concomitantly to an increase in the mRNA expression of the hepcidin suppressor erythroferrone (ERFE) in the bone marrow and the spleen. Compared with wild-type mice, Erfe-/- mice failed to adequately suppress hepcidin expression after infection with PbK. Importantly, the sustained production of hepcidin allowed by ERFE ablation was associated with decreased parasitemia, providing further evidence that transient iron restriction could be beneficial in the treatment of malaria. |
Menard, D.; Khim, N.; Beghain, J.; Adegnika, A. A.; Shafiul-Alam, M.; Amodu, O.; Rahim-Awab, G.; Barnadas, C.; Berry, A.; Boum, Y.; Bustos, M. D.; Cao, J.; Chen, J. H.; Collet, L.; Cui, L.; Thakur, G. D.; Dieye, A.; Djalle, D.; Dorkenoo, M. A.; Eboumbou-Moukoko, C. E.; Espino, F. E.; Fandeur, T.; Ferreira-da-Cruz, M. F.; Fola, A. A.; Fuehrer, H. P.; Hassan, A. M.; Herrera, S.; Hongvanthong, B.; Houze, S.; Ibrahim, M. L.; Jahirul-Karim, M.; Jiang, L.; Kano, S.; Ali-Khan, W.; Khanthavong, M.; Kremsner, P. G.; Lacerda, M.; Leang, R.; Leelawong, M.; Li, M.; Lin, K.; Mazarati, J. B.; Menard, S.; Morlais, I.; Muhindo-Mavoko, H.; Musset, L.; Na-Bangchang, K.; Nambozi, M.; Niare, K.; Noedl, H.; Ouedraogo, J. B.; Pillai, D. R.; Pradines, B.; Quang-Phuc, B.; Ramharter, M.; Randrianarivelojosia, M.; Sattabongkot, J.; Sheikh-Omar, A.; Silue, K. D.; Sirima, S. B.; Sutherland, C.; Syafruddin, D.; Tahar, R.; Tang, L. H.; Toure, O. A.; Tshibangu-wa-Tshibangu, P.; Vigan-Womas, I.; Warsame, M.; Wini, L.; Zakeri, S.; Kim, S.; Eam, R.; Berne, L.; Khean, C.; Chy, S.; Ken, M.; Loch, K.; Canier, L.; Duru, V.; Legrand, E.; Barale, J. C.; Stokes, B.; Straimer, J.; Witkowski, B.; Fidock, D. A.; Rogier, C.; Ringwald, P.; Ariey, F.; Mercereau-Puijalon, O. A Worldwide Map of Plasmodium falciparum K13-Propeller Polymorphisms Journal Article In: N Engl J Med, vol. 374, no. 25, pp. 2453-64, 2016, (Jun 23;374(25):2453-64. doi: 10.1056/NEJMoa1513137.). @article{b,BACKGROUND: Recent gains in reducing the global burden of malaria are threatened by the emergence of Plasmodium falciparum resistance to artemisinins. The discovery that mutations in portions of a P. falciparum gene encoding kelch (K13)-propeller domains are the major determinant of resistance has provided opportunities for monitoring such resistance on a global scale. METHODS: We analyzed the K13-propeller sequence polymorphism in 14,037 samples collected in 59 countries in which malaria is endemic. Most of the samples (84.5%) were obtained from patients who were treated at sentinel sites used for nationwide surveillance of antimalarial resistance. We evaluated the emergence and dissemination of mutations by haplotyping neighboring loci. RESULTS: We identified 108 nonsynonymous K13 mutations, which showed marked geographic disparity in their frequency and distribution. In Asia, 36.5% of the K13 mutations were distributed within two areas--one in Cambodia, Vietnam, and Laos and the other in western Thailand, Myanmar, and China--with no overlap. In Africa, we observed a broad array of rare nonsynonymous mutations that were not associated with delayed parasite clearance. The gene-edited Dd2 transgenic line with the A578S mutation, which expresses the most frequently observed African allele, was found to be susceptible to artemisinin in vitro on a ring-stage survival assay. CONCLUSIONS: No evidence of artemisinin resistance was found outside Southeast Asia and China, where resistance-associated K13 mutations were confined. The common African A578S allele was not associated with clinical or in vitro resistance to artemisinin, and many African mutations appear to be neutral. (Funded by Institut Pasteur Paris and others.). |
Menard, S.; Tchoufack, J. N.; Maffo, C. N.; Nsango, S. E.; Iriart, X.; Abate, L.; Tsapi, M. T.; Awono-Ambene, P. H.; Abega Mekongo, F. A.; Morlais, I.; Berry, A. Insight into k13-propeller gene polymorphism and ex vivo DHA-response profiles from Cameroonian isolates Journal Article In: Malar J, vol. 15, no. 1, pp. 572, 2016, (Nov 26;15(1):572.). @article{b,BACKGROUND: The spread of Plasmodium falciparum resistance to artemisinin derivatives in Southeast Asia is a major source of concern and the emergence of resistance in Africa would have dramatic consequences, by increasing malaria mortality and morbidity. It is therefore urgent to implement regular monitoring in sentinel sites in sub-Saharan Africa using robust and easy-to-implement tools. The prevalence of k13-propeller mutations and the phenotypic profiles are poorly known in sub-Saharan Africa. Here, the k13-propeller polymorphism was compared to both ex vivo susceptibility to DHA and early parasitological and clinical responses to artemisinin combination therapy (ACT). METHODS: Plasmodium falciparum isolates were collected in 2015 in Yaounde (Cameroon) from patients treated with dihydroartemisinin-piperaquine combination. Samples were analysed for their susceptibility to artemisinin using the k13-propeller sequencing, the ex vivo ring-stage survival assay, the in vivo parasite positive rate and the clinical statute at day 2. RESULTS: None of the collected isolates revealed the presence of resistance mutations in the k13-propeller sequence. The median ring-stage survival rate for all the 64 interpretable isolates after a 6-hour pulse of 700 nM dihydroartemisinin was low, 0.49% (IQR: 0-1.3). Total parasite clearance was observed for 87.5% of patients and the remaining parasitaemic isolates (12.5%) showed a high reduction of parasite load, ranging from 97.5 to 99.9%. Clinical symptoms disappeared in 92.8% of cases. CONCLUSION: This study demonstrated the absence of k13-resistant genotypes in P. falciparum isolates from Cameroon. Only synonymous mutations were found with a low prevalence (4.3%). A good association between k13 genotypes and the ex vivo ring-stage survival assay or parasitological and clinical data was obtained. These results give a baseline for the long-term monitoring of artemisinin derivative efficacy in Africa. |
Sanecka, A.; Yoshida, N.; Dougan, S. K.; Jackson, J.; Shastri, N.; Ploegh, H.; Blanchard, N.; Frickel, E. M. Transnuclear CD8 T cells specific for the immunodominant epitope Gra6 lower acute-phase Toxoplasma gondii burden Journal Article In: Immunology, 2016, (Jul 5. doi: 10.1111/imm.12643.). @article{b,We generated a CD8 T-cell receptor (TCR) transnuclear (TN) mouse specific to the Ld -restricted immunodominant epitope of GRA6 from Toxoplasma gondii as a source of cells to facilitate further investigation into the CD8 T-cell-mediated response against this pathogen. The TN T cells bound Ld -Gra6 tetramer and proliferated upon unspecific and peptide-specific stimulation. The TCR beta sequence of the Gra6-specific TN CD8 T cells is identical in its V- and J-region to the TCR-beta harboured by a hybridoma line generated in response to Gra6 peptide. Adoptively transferred Gra6 TN CD8 T cells proliferated upon Toxoplasma infection in vivo and exhibited an activated phenotype similar to host CD8 T cells specific to Gra6. The brain of Toxoplasma-infected mice carried Gra6 TN cells already at day 8 post-infection. Both Gra6 TN mice as well as adoptively transferred Gra6 TN cells were able to significantly reduce the parasite burden in the acute phase of Toxoplasma infection. Overall, the Gra6 TN mouse represents a functional tool to study the protective and immunodominant specific CD8 T-cell response to Toxoplasma in both the acute and the chronic phases of infection. |
2015 |
Joulia, R.; Gaudenzio, N.; Rodrigues, M.; Lopez, J.; Blanchard, N.; Valitutti, S.; Espinosa, E. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence Journal Article In: Nat Commun, vol. 6, pp. 6174, 2015, (Jan 28;6:6174. doi: 10.1038/ncomms7174.). @article{b,Mast cells are tissue-resident immune cells that play a key role in inflammation and allergy. Here we show that interaction of mast cells with antibody-targeted cells induces the polarized exocytosis of their granules resulting in a sustained exposure of effector enzymes, such as tryptase and chymase, at the cell-cell contact site. This previously unidentified mast cell effector mechanism, which we name the antibody-dependent degranulatory synapse (ADDS), is triggered by both IgE- and IgG-targeted cells. ADDSs take place within an area of cortical actin cytoskeleton clearance in the absence of microtubule organizing centre and Golgi apparatus repositioning towards the stimulating cell. Remarkably, IgG-mediated degranulatory synapses also occur upon contact with opsonized Toxoplasma gondii tachyzoites resulting in tryptase-dependent parasite death. Our results broaden current views of mast cell degranulation by revealing that human mast cells form degranulatory synapses with antibody-targeted cells and pathogens for dedicated secretion and defence. |
Lopez, J.; Bittame, A.; Massera, C.; Vasseur, V.; Effantin, G.; Valat, A.; Buaillon, C.; Allart, S.; Fox, B. A.; Rommereim, L. M.; Bzik, D. J.; Schoehn, G.; Weissenhorn, W.; Dubremetz, J. F.; Gagnon, J.; Mercier, C.; Cesbron-Delauw, M. F.; Blanchard, N. Intravacuolar Membranes Regulate CD8 T Cell Recognition of Membrane-Bound Toxoplasma gondii Protective Antigen Journal Article In: Cell Rep, 2015, (Nov 23. pii: S2211-1247(15)01288-7. doi: 10.1016/j.celrep.2015.11.001.). @article{b,Apicomplexa parasites such as Toxoplasma gondii target effectors to and across the boundary of their parasitophorous vacuole (PV), resulting in host cell subversion and potential presentation by MHC class I molecules for CD8 T cell recognition. The host-parasite interface comprises the PV limiting membrane and a highly curved, membranous intravacuolar network (IVN) of uncertain function. Here, using a cell-free minimal system, we dissect how membrane tubules are shaped by the parasite effectors GRA2 and GRA6. We show that membrane association regulates access of the GRA6 protective antigen to the MHC I pathway in infected cells. Although insertion of GRA6 in the PV membrane is key for immunogenicity, association of GRA6 with the IVN limits presentation and curtails GRA6-specific CD8 responses in mice. Thus, membrane deformations of the PV regulate access of antigens to the MHC class I pathway, and the IVN may play a role in immune modulation. |
Chauvin, P.; Menard, S.; Iriart, X.; Nsango, S. E.; Tchioffo, M. T.; Abate, L.; Awono-Ambene, P. H.; Morlais, I.; Berry, A. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaounde, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles Journal Article In: J Antimicrob Chemother, vol. 70, no. 9, pp. 2566-71, 2015, (Sep;70(9):2566-71. doi: 10.1093/jac/dkv160. Epub 2015 Jun 16.). @article{b,OBJECTIVES: To determine, 6 years after the adoption of intermittent preventive treatment of pregnant women with sulfadoxine/pyrimethamine (IPTp-SP) in Cameroon, (i) the polymorphism and prevalence of Plasmodium falciparum dihydrofolate reductase (pfdhfr) and dihydropteroate synthase (pfdhps) gene mutations associated with sulfadoxine/pyrimethamine resistance and (ii) the consequences of sulfadoxine/pyrimethamine use in the selection of pfdhfr/pfdhps alleles. METHODS: pfdhfr and pfdhps genes from P. falciparum isolates collected in Yaounde (Cameroon) from pregnant women with symptomatic malaria before taking IPTp-SP [SP- group (control) (n = 51)] or afterwards [SP+ group (n = 49)] were sequenced. RESULTS: The pfdhfr N51I, C59R, S108N triple mutant had a prevalence close to 100% (96/100) and no mutations at codons 50 and 164 were detected in either of the groups. The most frequent pfdhps mutation was A437G with a prevalence of 76.5% (39/51) in the SP- group, which was significantly higher in pregnant women who took sulfadoxine/pyrimethamine [95.9% (47/49)] (P = 0.012). Our study confirmed the presence of the pfdhps K540E mutation in Cameroon, but it remained rare. The prevalence of pfdhps A581G and A613S mutations had increased [5.9% (3/51) and 11.8% (6/51) in the control group, respectively] since the last studies in 2005. Surprisingly, the new pfdhps I431V mutation was detected, at a prevalence of 9.8% (5/51), and was found to be associated with other pfdhfr/pfdhps alleles to form an octuple N51I, C59R, S108N/I431V, S436A, A437G, A581G, A613S mutant. CONCLUSIONS: Significant changes were found in pfdhps polymorphism. In particular, we observed several parasites carrying eight mutations in pfdhfr/pfdhps genes, which are very susceptible to having a high level of resistance to sulfadoxine/pyrimethamine. |
Menard, S.; Ben Haddou, T.; Ramadani, A. P.; Ariey, F.; Iriart, X.; Beghain, J.; Bouchier, C.; Witkowski, B.; Berry, A.; Mercereau-Puijalon, O.; Benoit-Vical, F. Induction of Multidrug Tolerance in Plasmodium falciparum by Extended Artemisinin Pressure Journal Article In: Emerg Infect Dis, vol. 21, no. 10, pp. 1733-41, 2015, (Oct;21(10):1733-41. doi: 10.3201/eid2110.150682.). @article{b,Plasmodium falciparum resistance to artemisinin derivatives in Southeast Asia threatens global malaria control strategies. Whether delayed parasite clearance, which exposes larger parasite numbers to artemisinins for longer times, selects higher-grade resistance remains unexplored. We investigated whether long-lasting artemisinin pressure selects a novel multidrug-tolerance profile. Although 50% inhibitory concentrations for 10 antimalarial drugs tested were unchanged, drug-tolerant parasites showed higher recrudescence rates for endoperoxides, quinolones, and an antifolate, including partner drugs of recommended combination therapies, but remained susceptible to atovaquone. Moreover, the age range of intraerythrocytic stages able to resist artemisinin was extended to older ring forms and trophozoites. Multidrug tolerance results from drug-induced quiescence, which enables parasites to survive exposure to unrelated antimalarial drugs that inhibit a variety of metabolic pathways. This novel resistance pattern should be urgently monitored in the field because this pattern is not detected by current assays and represents a major threat to antimalarial drug policy. |
Blanchard, N.; Dunay, I. R.; Schluter, D. Persistence of Toxoplasma gondii in the central nervous system: a fine tuned balance between the parasite, the brain and the immune system Journal Article In: Parasite Immunol, 2015, (Jan 9. doi: 10.1111/pim.12173.). @article{b,Upon infection of humans and animals with Toxoplasma gondii, the parasites persist as intraneuronal cysts that are controlled, but not eliminated by the immune system. In particular, intracerebral T cells are crucial in the control of T. gondii infection and are supported by essential functions from other leukocyte populations. Additionally, brain-resident cells including astrocytes, microglia and neurons contribute to the intracerebral immune response by the production of cytokines, chemokines and expression of immunoregulatory cell surface molecules, such as major histocompatibility (MHC) antigens. However, the in vivo behaviour of these individual cell populations, specifically their interaction during cerebral toxoplasmosis, remains to be elucidated. We discuss here what is known about the function of T cells, recruited myeloid cells and brain-resident cells, with particular emphasis on the potential cross-regulation of these cell populations, in governing cerebral toxoplasmosis. This article is protected by copyright. All rights reserved. |
2013 |
Witkowski, B.; Khim, N.; Chim, P.; Kim, S.; Ke, S.; Kloeung, N.; Chy, S.; Duong, S.; Leang, R.; Ringwald, P.; Dondorp, A. M.; Tripura, R.; Benoit-Vical, F.; Berry, A.; Gorgette, O.; Ariey, F.; Barale, J. C.; Mercereau-Puijalon, O.; Menard, D. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia Journal Article In: Antimicrob Agents Chemother, vol. 57, no. 2, pp. 914-23, 2013, (Feb;57(2):914-23. doi: 10.1128/AAC.01868-12. Epub 2012 Dec 3.). @article{b,The declining efficacy of artemisinin derivatives against Plasmodium falciparum in western Cambodia is a major concern. The knowledge gap in the understanding of the mechanisms involved hampers designing monitoring tools. Here, we culture-adapted 20 isolates from Pailin and Ratanakiri (areas of artemisinin resistance and susceptibility in western and eastern Cambodia, respectively) and studied their in vitro response to dihydroartemisinin. No significant difference between the two sets of isolates was observed in the classical isotopic test. However, a 6-h pulse exposure to 700 nM dihydroartemisinin (ring-stage survival assay -RSA]) revealed a clear-cut geographic dichotomy. The survival rate of exposed ring-stage parasites (ring stages) was 17-fold higher in isolates from Pailin (median, 13.5%) than in those from Ratanakiri (median, 0.8%), while exposed mature stages were equally and highly susceptible (0.6% and 0.7%, respectively). Ring stages survived drug exposure by cell cycle arrest and resumed growth upon drug withdrawal. The reduced susceptibility to artemisinin in Pailin appears to be associated with an altered in vitro phenotype of ring stages from Pailin in the RSA. |
Feliu, V.; Vasseur, V.; Grover, H. S.; Chu, H. H.; Brown, M. J.; Wang, J.; Boyle, J. P.; Robey, E. A.; Shastri, N.; Blanchard, N. Location of the CD8 T Cell Epitope within the Antigenic Precursor Determines Immunogenicity and Protection against the Toxoplasma gondii Parasite Journal Article In: PLoS pathogens, vol. 9, no. 6, pp. e1003449, 2013, (Jun;9(6):e1003449. doi: 10.1371/journal.ppat.1003449. Epub 2013 Jun 20.). @article{b,CD8 T cells protect the host from disease caused by intracellular pathogens, such as the Toxoplasma gondii (T. gondii) protozoan parasite. Despite the complexity of the T. gondii proteome, CD8 T cell responses are restricted to only a small number of peptide epitopes derived from a limited set of antigenic precursors. This phenomenon is known as immunodominance and is key to effective vaccine design. However, the mechanisms that determine the immunogenicity and immunodominance hierarchy of parasite antigens are not well understood. Here, using genetically modified parasites, we show that parasite burden is controlled by the immunodominant GRA6-specific CD8 T cell response but not by responses to the subdominant GRA4- and ROP7-derived epitopes. Remarkably, optimal processing and immunodominance were determined by the location of the peptide epitope at the C-terminus of the GRA6 antigenic precursor. In contrast, immunodominance could not be explained by the peptide affinity for the MHC I molecule or the frequency of T cell precursors in the naive animals. Our results reveal the molecular requirements for optimal presentation of an intracellular parasite antigen and for eliciting protective CD8 T cells. |
Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A. C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; Lim, P.; Leang, R.; Duong, S.; Sreng, S.; Suon, S.; Chuor, C. M.; Bout, D. M.; Menard, S.; Rogers, W. O.; Genton, B.; Fandeur, T.; Miotto, O.; Ringwald, P.; Le Bras, J.; Berry, A.; Barale, J. C.; Fairhurst, R. M.; Benoit-Vical, F.; Mercereau-Puijalon, O.; Menard, D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria Journal Article In: Nature, 2013, (Dec 18. doi: 10.1038/nature12876.). @article{,Plasmodium falciparum resistance to artemisinin derivatives in southeast Asia threatens malaria control and elimination activities worldwide. To monitor the spread of artemisinin resistance, a molecular marker is urgently needed. Here, using whole-genome sequencing of an artemisinin-resistant parasite line from Africa and clinical parasite isolates from Cambodia, we associate mutations in the PF3D7_1343700 kelch propeller domain ('K13-propeller') with artemisinin resistance in vitro and in vivo. Mutant K13-propeller alleles cluster in Cambodian provinces where resistance is prevalent, and the increasing frequency of a dominant mutant K13-propeller allele correlates with the recent spread of resistance in western Cambodia. Strong correlations between the presence of a mutant allele, in vitro parasite survival rates and in vivo parasite clearance rates indicate that K13-propeller mutations are important determinants of artemisinin resistance. K13-propeller polymorphism constitutes a useful molecular marker for large-scale surveillance efforts to contain artemisinin resistance in the Greater Mekong Subregion and prevent its global spread. |
2012 |
Menard, S.; Morlais, I.; Tahar, R.; Sayang, C.; Mayengue, P. I.; Iriart, X.; Benoit-Vical, F.; Lemen, B.; Magnaval, J. F.; Awono-Ambene, P.; Basco, L. K.; Berry, A. Molecular monitoring of plasmodium falciparum drug susceptibility at the time of the introduction of artemisinin-based combination therapy in Yaounde, Cameroon: implications for the future Journal Article In: Malaria journal, vol. 11, pp. 113, 2012, (Apr 12;11:113.). @article{b,BACKGROUND: Regular monitoring of the levels of anti-malarial resistance of Plasmodium falciparum is an essential policy to adapt therapy and improve malaria control. This monitoring can be facilitated by using molecular tools, which are easier to implement than the classical determination of the resistance phenotype. In Cameroon, chloroquine (CQ), previously the first-line therapy for uncomplicated malaria was officially withdrawn in 2002 and replaced initially by amodiaquine (AQ) monotherapy. Then, artemisinin-based combination therapy (ACT), notably artesunate-amodiaquine (AS-AQ) or artemether-lumefantrine (AL), was gradually introduced in 2004. This situation raised the question of the evolution of P. falciparum resistance molecular markers in Yaounde, a highly urbanized Cameroonian city. METHODS: The genotype of pfcrt 72 and 76 and pfmdr1 86 alleles and pfmdr1 copy number were determined using real-time PCR in 447 P. falciparum samples collected between 2005 and 2009. RESULTS: This study showed a high prevalence of parasites with mutant pfcrt 76 (83%) and pfmdr1 86 (93%) codons. On the contrary, no mutations in the pfcrt 72 codon and no samples with duplication of the pfmdr1 gene were observed. CONCLUSION: The high prevalence of mutant pfcrt 76T and pfmdr1 86Y alleles might be due to the choice of alternative drugs (AQ and AS-AQ) known to select such genotypes. Mutant pfcrt 72 codon was not detected despite the prolonged use of AQ either as monotherapy or combined with artesunate. The absence of pfmdr1 multicopies suggests that AL would still remain efficient. The limited use of mefloquine or the predominance of mutant pfmdr1 86Y codon could explain the lack of pfmdr1 amplification. Indeed, this mutant codon is rarely associated with duplication of pfmdr1 gene. In Cameroon, the changes of therapeutic strategies and the simultaneous use of several formulations of ACT or other anti-malarials that are not officially recommended result in a complex selective pressure, rendering the prediction of the evolution of P. falciparum resistance difficult. This public health problem should lead to increased vigilance and regular monitoring. |
2011 |
Cebrian, I.; Visentin, G.; Blanchard, N.; Jouve, M.; Bobard, A.; Moita, C.; Enninga, J.; Moita, L. F.; Amigorena, S.; Savina, A. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells Journal Article In: Cell, vol. 147, no. 6, pp. 1355-68, 2011, (Dec 9;147(6):1355-68.). @article{b,Antigen (Ag) crosspresentation by dendritic cells (DCs) involves the presentation of internalized Ags on MHC class I molecules to initiate CD8+ T cell-mediated immunity in response to certain pathogens and tumor cells. Here, we identify the SNARE Sec22b as a specific regulator of Ag crosspresentation. Sec22b localizes to the ER-Golgi intermediate compartment (ERGIC) and pairs to the plasma membrane SNARE syntaxin 4, which is present in phagosomes (Phgs). Depletion of Sec22b inhibits the recruitment of ER-resident proteins to Phgs and to the vacuole containing the Toxoplasma gondii parasite. In Sec22b-deficient DCs, crosspresentation is compromised after Ag phagocytosis or endocytosis and after invasion by T. gondii. Sec22b silencing inhibited Ag export to the cytosol and increased phagosomal degradation by accelerating lysosomal recruitment. Our findings provide insight into an intracellular traffic pathway required for crosspresentation and show that Sec22b-dependent recruitment of ER proteins to Phgs critically influences phagosomal functions in DCs. |
2008 |
Blanchard, N.; Gonzalez, F.; Schaeffer, M.; Joncker, N. T.; Cheng, T.; Shastri, A. J.; Robey, E. A.; Shastri, N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum Journal Article In: Nat Immunol, vol. 9, no. 8, pp. 937-44, 2008, (NLM In-Process Journal Article Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't DEP - 20080629). @article{b,The parasite Toxoplasma gondii replicates in a specialized intracellular vacuole and causes disease in many species. Protection from toxoplasmosis is mediated by CD8(+) T cells, but the T. gondii antigens and host genes required for eliciting protective immunity are poorly defined. Here we identified GRA6, a polymorphic protein secreted in the parasitophorous vacuole, as the source of the immunodominant and protective decapeptide HF10 presented by the H-2L(d) major histocompatibility complex class I molecule. Presentation of the HF10-H-2L(d) ligand required proteolysis by ERAAP, the endoplasmic reticulum aminopeptidase associated with antigen processing. Consequently, expansion of protective CD8(+) T cell populations was impaired in T. gondii-infected ERAAP-deficient mice, which were more susceptible to toxoplasmosis. Thus, endoplasmic reticulum proteolysis is critical for eliciting protective immunity to a vacuolar parasite. |
Impact on the society
Our field work in Africa will provide valuable information on the spread of Plasmodium isolates that are resistant to artemisinin derivatives as well as to other drugs effective for treating malaria.
These data should allow us to swiftly develop new control strategies in order to limit the burden of these resistant clones. A better understanding of the biology of resistant parasites is also essential to devise new pharmacological strategies.
Our fundamental work on immunity to Toxoplasma gondii and Plasmodium has the potential to uncover novel pathways and/or molecules by which intracellular parasites manipulate presentation of their own antigens, modulate immune responses and, more generally, subvert their host.
Our studies could help identify or down-select new vaccine targets. They may also suggest approaches to use CD8 T cells in order to eradicate T. gondii cysts, against which there is currently no effective pharmacological treatment.
Collaborations
Local
Dr Chrystelle Bonnart, N. Vergnolle’s team, Institute for Research on Digestive Health, Toulouse
Drs Gilles Dietrich & Nicolas Cenac, Institute for Research on Digestive Health, Toulouse
Dr Nicolas Fazilleau, Infinity
Drs Roland Liblau & Abdelhadi Saoudi, Infinity
Dr Elsa Suberbielle, D. Dunia’s team, Infinity
Drs Virginie Mieulet & Manuel Diaz-Munoz, Infinity
National
Dr Antoine Claessens, LPHI, Montpellier
Dr Sylvie Garcia, A. Sherf’s team, Pasteur Institute, Paris
Dr Mohamed-Ali Hakimi, IAB, Grenoble
Dr Sabrina Marion, CIIL / Pasteur Institute, Lille
Dr Isabelle Morlais, MIVEGEC, Montpellier
Dr Sandrine Marquet, TAGC lab, Marseille
Dr David Blum, LiLNCog, Lille
Dr Guillaume Dorothée, Saint-Antoine Research Center (CRSA), Paris
Drs Gilles Marodon & Olivier Silvie, CIMI Paris
International
Pr Parfait Awono-Ambene, Yaoundé, Cameroon
Drs Ignacio Cebrian & Luis Mayorga, IHEM-CONICET, University of Cuyo, Mendoza, Argentina
Dr Klaas van Gisbergen, Champalimaud Centre for the Unknown, Lisbon, Portugal
Pr Ellen Robey, University of California, Berkeley, USA
Dr Joost Smolders, Erasmus MC, Rotterdam, The Netherlands
Alumni
Aboubacar Coulibaly PhD student 2021-2024
Alexis Audibert PhD student 2021-2024
Marcy Belloy Engineer 2017-2019 / PhD Student 2019-2023
Eléna Charpentier, Pharm.D. / PhD student 2017-2020
Aurore Lebourg Engineer 2018-2020
Mélissa Mairet-Khedim PhD student 2016-2020
Ali Hassan PhD student 2016-2019
Anna Salvioni PhD student 2016-2019
Nian-Zhang Zhang Visiting Scholar from Lanzhou Veterinary Research Institute, China, 2017-2019
Julien Santi-Rocca Post-doc 2015-2017
Myriam Wlodarczyk Post-doc 2012-2017
Marion Draheim PhD Student, 2014-2016
Virginie Vasseur Engineer, 2010-2016
Francesca de Giorgi Unipharma Graduate Program / Erasmus student, 2016
Célia Buaillon PhD student 2012-2016
Jodie Lopez PhD student 2012-2015
Nestor Guerrero Post-doc, 2013-2015
Giulia Fornabaio European Unipharma Graduate Program student, 2015
Valeria Bellini European Unipharma Graduate Program student, 2012-2013
Virginie Feliu Engineer
Rose-Anne Lavergne, MD Interne 2011-2013
Sophie Blanié Post-doc, 2010-2012