2024
|
Tournezy, Jeflie; Léger, Claire; Klonjkowski, Bernard; Gonzalez-Dunia, Daniel; Szelechowski, Marion; Garenne, André; Mathis, Stéphane; Chevallier, Stéphanie; Masson, Gwendal Le The Neuroprotective Effect of the X Protein of Orthobornavirus Bornaense Type 1 in Amyotrophic Lateral Sclerosis Article de journal Dans: IJMS, vol. 25, no. 23, 2024, ISSN: 1422-0067. @article{Tournezy2024,
title = {The Neuroprotective Effect of the X Protein of Orthobornavirus Bornaense Type 1 in Amyotrophic Lateral Sclerosis},
author = {Jeflie Tournezy and Claire Léger and Bernard Klonjkowski and Daniel Gonzalez-Dunia and Marion Szelechowski and André Garenne and Stéphane Mathis and Stéphanie Chevallier and Gwendal Le Masson},
doi = {10.3390/ijms252312789},
issn = {1422-0067},
year = {2024},
date = {2024-12-00},
urldate = {2024-12-00},
journal = {IJMS},
volume = {25},
number = {23},
publisher = {MDPI AG},
abstract = {<jats:p>In amyotrophic lateral sclerosis (ALS), early mitochondrial dysfunction may contribute to progressive motor neuron loss. Remarkably, the ectopic expression of the Orthobornavirus bornaense type 1 (BoDV-1) X protein in mitochondria blocks apoptosis and protects neurons from degeneration. Therefore, this study examines the neuroprotective effects of X protein in an ALS mouse model. We first tested in vitro the effect of the X-derived peptide (PX3) on motoneurons primary cultures of SOD1G93A mice. The total intracellular adenosine triphosphate (ATP) content was measured after incubation of the peptide. We next tested in vivo the intramuscular injection of X protein using a canine viral vector (CAV2-X) and PX3 intranasal administrations in SOD1G93A mice. Disease onset and progression were assessed through rotarod performance, functional motor unit analysis via electrophysiology, and motor neuron survival by immunohistochemistry. The results showed that in vitro PX3 restored the ATP level in SOD1G93A motor neurons. In vivo, treated mice demonstrated better motor performance, preserved motor units, and higher motor neuron survival. Although life expectancy was not extended in this severe mouse model of motor neuron degeneration, the present findings clearly demonstrate the neuroprotective potential of X protein in a model of ALS. We are convinced that further studies may improve the therapeutic impact of X protein with optimized administration methods.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>In amyotrophic lateral sclerosis (ALS), early mitochondrial dysfunction may contribute to progressive motor neuron loss. Remarkably, the ectopic expression of the Orthobornavirus bornaense type 1 (BoDV-1) X protein in mitochondria blocks apoptosis and protects neurons from degeneration. Therefore, this study examines the neuroprotective effects of X protein in an ALS mouse model. We first tested in vitro the effect of the X-derived peptide (PX3) on motoneurons primary cultures of SOD1G93A mice. The total intracellular adenosine triphosphate (ATP) content was measured after incubation of the peptide. We next tested in vivo the intramuscular injection of X protein using a canine viral vector (CAV2-X) and PX3 intranasal administrations in SOD1G93A mice. Disease onset and progression were assessed through rotarod performance, functional motor unit analysis via electrophysiology, and motor neuron survival by immunohistochemistry. The results showed that in vitro PX3 restored the ATP level in SOD1G93A motor neurons. In vivo, treated mice demonstrated better motor performance, preserved motor units, and higher motor neuron survival. Although life expectancy was not extended in this severe mouse model of motor neuron degeneration, the present findings clearly demonstrate the neuroprotective potential of X protein in a model of ALS. We are convinced that further studies may improve the therapeutic impact of X protein with optimized administration methods.</jats:p> |
Gourin, Claire; Flores, Thelma; Mafi, Sarah; Malnou, Cécile; Alain, Sophie; Hantz, Sébastien; Ligat, Gaëtan [Challenges and advances in the management of HCMV infections] Article de journal Dans: Virologie (Montrouge), vol. 28, no. 5, p. 309–325, 2024, ISSN: 1267-8694. @article{pmid39498798b,
title = {[Challenges and advances in the management of HCMV infections]},
author = {Claire Gourin and Thelma Flores and Sarah Mafi and Cécile Malnou and Sophie Alain and Sébastien Hantz and Gaëtan Ligat},
doi = {10.1684/vir.2024.1063},
issn = {1267-8694},
year = {2024},
date = {2024-10-01},
urldate = {2024-10-01},
journal = {Virologie (Montrouge)},
volume = {28},
number = {5},
pages = {309--325},
abstract = {Human cytomegalovirus (HCMV) is one of the most important causes of complications in immunocompromised patients and congenital infections. HCMV could also represent an interesting target for treatment to limit the progression of glioblastoma, a highly aggressive tumor. Ganciclovir, foscarnet and cidofovir, which interfere with the activity of the viral polymerase pUL54, are widely used in the treatment of transplant patients. However, their use in pregnant women remains limited or even contraindicated. On the other hand, hyperimmune immunoglobulins and valaciclovir have been shown to have a protective effect on the fetus. However, the toxicity of these treatments and the emergence of resistance mean that new therapeutic strategies need to be identified. Letermovir and maribavir have been developed to inhibit new targets, respectively the terminase complex and UL97 protein kinase. Their respective indications are the prevention of HCMV infection in haematopoietic stem cell transplant patients and the treatment of refractory HCMV infections. Finally, with the development of mRNA vaccines, the hope of one day seeing a prophylactic HCMV vaccine has never been greater. New therapeutic approaches are also being explored, but they still require extensive preclinical and clinical evaluation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Human cytomegalovirus (HCMV) is one of the most important causes of complications in immunocompromised patients and congenital infections. HCMV could also represent an interesting target for treatment to limit the progression of glioblastoma, a highly aggressive tumor. Ganciclovir, foscarnet and cidofovir, which interfere with the activity of the viral polymerase pUL54, are widely used in the treatment of transplant patients. However, their use in pregnant women remains limited or even contraindicated. On the other hand, hyperimmune immunoglobulins and valaciclovir have been shown to have a protective effect on the fetus. However, the toxicity of these treatments and the emergence of resistance mean that new therapeutic strategies need to be identified. Letermovir and maribavir have been developed to inhibit new targets, respectively the terminase complex and UL97 protein kinase. Their respective indications are the prevention of HCMV infection in haematopoietic stem cell transplant patients and the treatment of refractory HCMV infections. Finally, with the development of mRNA vaccines, the hope of one day seeing a prophylactic HCMV vaccine has never been greater. New therapeutic approaches are also being explored, but they still require extensive preclinical and clinical evaluation. |
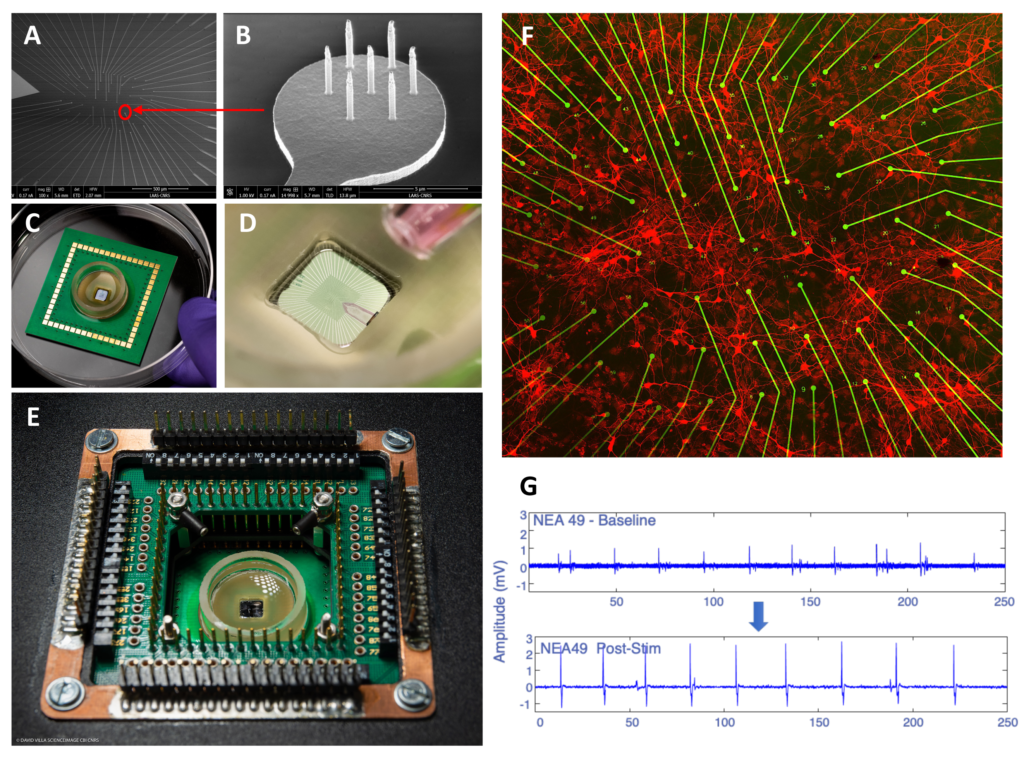
Bettamin, Luca; Mathieu, Fabrice; Marty, Florent H.; Blatche, Marie Charline; Gonzalez‐Dunia, Daniel; Suberbielle, Elsa; Larrieu, Guilhem Real‐Time and High‐Resolution Monitoring of Neuronal Electrical Activity and pH Variations Based on the Co‐Integration of Nanoelectrodes and Chem‐FinFETs Article de journal Dans: Small, vol. 20, no. 27, 2024, ISSN: 1613-6829. @article{Bettamin2024b,
title = {Real‐Time and High‐Resolution Monitoring of Neuronal Electrical Activity and pH Variations Based on the Co‐Integration of Nanoelectrodes and Chem‐FinFETs},
author = {Luca Bettamin and Fabrice Mathieu and Florent H. Marty and Marie Charline Blatche and Daniel Gonzalez‐Dunia and Elsa Suberbielle and Guilhem Larrieu},
doi = {10.1002/smll.202309055},
issn = {1613-6829},
year = {2024},
date = {2024-07-00},
urldate = {2024-07-00},
journal = {Small},
volume = {20},
number = {27},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:p>Developing new approaches amenable to the measurement of neuronal physiology in real‐time is a very active field of investigation, as it will offer improved methods to assess the impact of diverse insults on neuronal homeostasis. Here, the development of an in vitro bio platform is reported which can record the electrical activity of cultured primary rat cortical neurons with extreme sensitivity, while simultaneously tracking the localized changes in the pH of the culture medium. This bio platform features passive vertical nanoprobes with ultra‐high signal resolution (several mV amplitude ranges) and Chem‐FinFETs (pH sensitivity of sub‐0.1 pH units), covering an area as little as a neuronal soma. These multi‐sensing units are arranged in an array to probe both chemically and electrically an equivalent surface of ≈ 0.5 mm<jats:sup>2</jats:sup>. A homemade setup is also developed which allows recording of multiplexed data in real‐time (10 ps range) from the active chem‐sensors and passive electrodes and which is used to operate the platform. Finally, a proof‐of‐concept is presented for a neuro‐relevant application, by investigating the effect on neuronal activity of Amyloid beta oligomers, the main toxic peptide in Alzheimer's Disease, which reveals that exposure to amyloid beta oligomers modify the amplitude, but not the frequency, of neuronal firing, without any detectable changes in pH values along this process.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Developing new approaches amenable to the measurement of neuronal physiology in real‐time is a very active field of investigation, as it will offer improved methods to assess the impact of diverse insults on neuronal homeostasis. Here, the development of an in vitro bio platform is reported which can record the electrical activity of cultured primary rat cortical neurons with extreme sensitivity, while simultaneously tracking the localized changes in the pH of the culture medium. This bio platform features passive vertical nanoprobes with ultra‐high signal resolution (several mV amplitude ranges) and Chem‐FinFETs (pH sensitivity of sub‐0.1 pH units), covering an area as little as a neuronal soma. These multi‐sensing units are arranged in an array to probe both chemically and electrically an equivalent surface of ≈ 0.5 mm<jats:sup>2</jats:sup>. A homemade setup is also developed which allows recording of multiplexed data in real‐time (10 ps range) from the active chem‐sensors and passive electrodes and which is used to operate the platform. Finally, a proof‐of‐concept is presented for a neuro‐relevant application, by investigating the effect on neuronal activity of Amyloid beta oligomers, the main toxic peptide in Alzheimer's Disease, which reveals that exposure to amyloid beta oligomers modify the amplitude, but not the frequency, of neuronal firing, without any detectable changes in pH values along this process.</jats:p> |
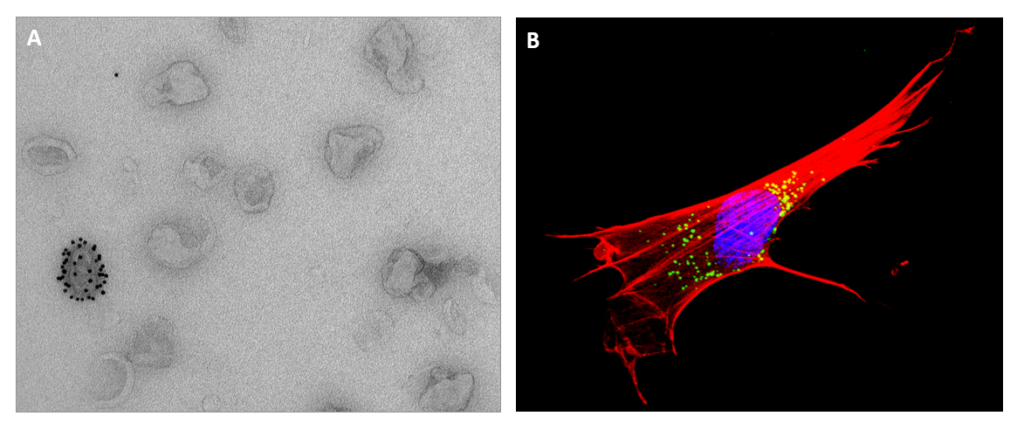
Welsh, Joshua A.; Goberdhan, Deborah C. I.; O'Driscoll, Lorraine; Buzas, Edit I.; Blenkiron, Cherie; Bussolati, Benedetta; Cai, Houjian; Vizio, Dolores Di; Driedonks, Tom A. P.; Erdbrügger, Uta; Falcon‐Perez, Juan M.; Fu, Qing‐Ling; Hill, Andrew F.; Lenassi, Metka; Lim, Sai Kiang; Mahoney, Mỹ G.; Mohanty, Sujata; Möller, Andreas; Nieuwland, Rienk; Ochiya, Takahiro; Sahoo, Susmita; Torrecilhas, Ana C.; Zheng, Lei; Zijlstra, Andries; Abuelreich, Sarah; Bagabas, Reem; Bergese, Paolo; Bridges, Esther M.; Brucale, Marco; Burger, Dylan; Carney, Randy P.; Cocucci, Emanuele; Crescitelli, Rossella; Hanser, Edveena; Harris, Adrian L.; Haughey, Norman J.; Hendrix, An; Ivanov, Alexander R.; Jovanovic‐Talisman, Tijana; Kruh‐Garcia, Nicole A.; Faustino, Vroniqa Ku'ulei‐Lyn; Kyburz, Diego; Lässer, Cecilia; Lennon, Kathleen M.; Lötvall, Jan; Maddox, Adam L.; Martens‐Uzunova, Elena S.; Mizenko, Rachel R.; Newman, Lauren A.; Ridolfi, Andrea; Rohde, Eva; Rojalin, Tatu; Rowland, Andrew; Saftics, Andras; Sandau, Ursula S.; Saugstad, Julie A.; Shekari, Faezeh; Swift, Simon; Ter‐Ovanesyan, Dmitry; Tosar, Juan P.; Useckaite, Zivile; Valle, Francesco; Varga, Zoltan; van der Pol, Edwin; van Herwijnen, Martijn J. C.; Wauben, Marca H. M.; Wehman, Ann M.; Williams, Sarah; Zendrini, Andrea; Zimmerman, Alan J.; and Clotilde Théry,; Witwer, Kenneth W. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches Article de journal Dans: J of Extracellular Vesicle, vol. 13, no. 2, 2024, ISSN: 2001-3078. @article{Welsh2024,
title = {Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches},
author = {Joshua A. Welsh and Deborah C. I. Goberdhan and Lorraine O'Driscoll and Edit I. Buzas and Cherie Blenkiron and Benedetta Bussolati and Houjian Cai and Dolores Di Vizio and Tom A. P. Driedonks and Uta Erdbrügger and Juan M. Falcon‐Perez and Qing‐Ling Fu and Andrew F. Hill and Metka Lenassi and Sai Kiang Lim and Mỹ G. Mahoney and Sujata Mohanty and Andreas Möller and Rienk Nieuwland and Takahiro Ochiya and Susmita Sahoo and Ana C. Torrecilhas and Lei Zheng and Andries Zijlstra and Sarah Abuelreich and Reem Bagabas and Paolo Bergese and Esther M. Bridges and Marco Brucale and Dylan Burger and Randy P. Carney and Emanuele Cocucci and Rossella Crescitelli and Edveena Hanser and Adrian L. Harris and Norman J. Haughey and An Hendrix and Alexander R. Ivanov and Tijana Jovanovic‐Talisman and Nicole A. Kruh‐Garcia and Vroniqa Ku'ulei‐Lyn Faustino and Diego Kyburz and Cecilia Lässer and Kathleen M. Lennon and Jan Lötvall and Adam L. Maddox and Elena S. Martens‐Uzunova and Rachel R. Mizenko and Lauren A. Newman and Andrea Ridolfi and Eva Rohde and Tatu Rojalin and Andrew Rowland and Andras Saftics and Ursula S. Sandau and Julie A. Saugstad and Faezeh Shekari and Simon Swift and Dmitry Ter‐Ovanesyan and Juan P. Tosar and Zivile Useckaite and Francesco Valle and Zoltan Varga and Edwin van der Pol and Martijn J. C. van Herwijnen and Marca H. M. Wauben and Ann M. Wehman and Sarah Williams and Andrea Zendrini and Alan J. Zimmerman and and Clotilde Théry and Kenneth W. Witwer},
doi = {10.1002/jev2.12404},
issn = {2001-3078},
year = {2024},
date = {2024-02-00},
urldate = {2024-02-00},
journal = {J of Extracellular Vesicle},
volume = {13},
number = {2},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:p>Extracellular vesicles (EVs), through their complex cargo, can reflect the state of their cell of origin and change the functions and phenotypes of other cells. These features indicate strong biomarker and therapeutic potential and have generated broad interest, as evidenced by the steady year‐on‐year increase in the numbers of scientific publications about EVs. Important advances have been made in EV metrology and in understanding and applying EV biology. However, hurdles remain to realising the potential of EVs in domains ranging from basic biology to clinical applications due to challenges in EV nomenclature, separation from non‐vesicular extracellular particles, characterisation and functional studies. To address the challenges and opportunities in this rapidly evolving field, the International Society for Extracellular Vesicles (ISEV) updates its ‘Minimal Information for Studies of Extracellular Vesicles’, which was first published in 2014 and then in 2018 as MISEV2014 and MISEV2018, respectively. The goal of the current document, MISEV2023, is to provide researchers with an updated snapshot of available approaches and their advantages and limitations for production, separation and characterisation of EVs from multiple sources, including cell culture, body fluids and solid tissues. In addition to presenting the latest state of the art in basic principles of EV research, this document also covers advanced techniques and approaches that are currently expanding the boundaries of the field. MISEV2023 also includes new sections on EV release and uptake and a brief discussion of in vivo approaches to study EVs. Compiling feedback from ISEV expert task forces and more than 1000 researchers, this document conveys the current state of EV research to facilitate robust scientific discoveries and move the field forward even more rapidly.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Extracellular vesicles (EVs), through their complex cargo, can reflect the state of their cell of origin and change the functions and phenotypes of other cells. These features indicate strong biomarker and therapeutic potential and have generated broad interest, as evidenced by the steady year‐on‐year increase in the numbers of scientific publications about EVs. Important advances have been made in EV metrology and in understanding and applying EV biology. However, hurdles remain to realising the potential of EVs in domains ranging from basic biology to clinical applications due to challenges in EV nomenclature, separation from non‐vesicular extracellular particles, characterisation and functional studies. To address the challenges and opportunities in this rapidly evolving field, the International Society for Extracellular Vesicles (ISEV) updates its ‘Minimal Information for Studies of Extracellular Vesicles’, which was first published in 2014 and then in 2018 as MISEV2014 and MISEV2018, respectively. The goal of the current document, MISEV2023, is to provide researchers with an updated snapshot of available approaches and their advantages and limitations for production, separation and characterisation of EVs from multiple sources, including cell culture, body fluids and solid tissues. In addition to presenting the latest state of the art in basic principles of EV research, this document also covers advanced techniques and approaches that are currently expanding the boundaries of the field. MISEV2023 also includes new sections on EV release and uptake and a brief discussion of in vivo approaches to study EVs. Compiling feedback from ISEV expert task forces and more than 1000 researchers, this document conveys the current state of EV research to facilitate robust scientific discoveries and move the field forward even more rapidly.</jats:p> |
2023
|
Malnou, Cécile E.; Ligat, Gaëtan Editorial: Recent highlights in the development of therapeutic antiviral strategies Article de journal Dans: Front. Microbiol., vol. 14, 2023, ISSN: 1664-302X. @article{Malnou2023,
title = {Editorial: Recent highlights in the development of therapeutic antiviral strategies},
author = {Cécile E. Malnou and Gaëtan Ligat},
doi = {10.3389/fmicb.2023.1338999},
issn = {1664-302X},
year = {2023},
date = {2023-12-05},
urldate = {2023-12-05},
journal = {Front. Microbiol.},
volume = {14},
publisher = {Frontiers Media SA},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Martin, Charlène; Ligat, Gaëtan; Malnou, Cécile E. The Yin and the Yang of extracellular vesicles during viral infections Article de journal Dans: Biomedical Journal, 2023, ISSN: 2319-4170. @article{Martin2023,
title = {The Yin and the Yang of extracellular vesicles during viral infections},
author = {Charlène Martin and Gaëtan Ligat and Cécile E. Malnou},
doi = {10.1016/j.bj.2023.100659},
issn = {2319-4170},
year = {2023},
date = {2023-09-00},
urldate = {2023-09-00},
journal = {Biomedical Journal},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Fourgeaud, Jacques; Magny, Jean-François; Couderc, Sophie; Garcia, Patricia; Maillotte, Anne-Marie; Benard, Melinda; Pinquier, Didier; Minodier, Philippe; Astruc, Dominique; Patural, Hugues; Ugolin, Melissa; Parat, Sophie; Guillois, Bernard; Garenne, Armelle; Guilleminot, Tiffany; Parodi, Marine; Bussières, Laurence; Ville, Yves; Leruez-Ville, Marianne Clinical Value of Serial Quantitative Analysis of Cytomegalovirus DNA in Blood and Saliva Over the First 24 Months of Life in Congenital Infection: The French Cymepedia Cohort Article de journal Dans: The Journal of Pediatrics, vol. 253, p. 197–204.e5, 2023, ISSN: 0022-3476. @article{Fourgeaud2023,
title = {Clinical Value of Serial Quantitative Analysis of Cytomegalovirus DNA in Blood and Saliva Over the First 24 Months of Life in Congenital Infection: The French Cymepedia Cohort},
author = {Jacques Fourgeaud and Jean-François Magny and Sophie Couderc and Patricia Garcia and Anne-Marie Maillotte and Melinda Benard and Didier Pinquier and Philippe Minodier and Dominique Astruc and Hugues Patural and Melissa Ugolin and Sophie Parat and Bernard Guillois and Armelle Garenne and Tiffany Guilleminot and Marine Parodi and Laurence Bussières and Yves Ville and Marianne Leruez-Ville},
doi = {10.1016/j.jpeds.2022.09.040},
issn = {0022-3476},
year = {2023},
date = {2023-02-00},
urldate = {2023-02-00},
journal = {The Journal of Pediatrics},
volume = {253},
pages = {197--204.e5},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2022
|
Martin, Charlène; Bergamelli, Mathilde; Malnou, Cécile E.; D'Angelo, Gisela Placental extracellular vesicles in maternal-fetal communication during pregnancy Article de journal Dans: Biochem Soc Trans, vol. 50, no. 6, p. 1785–1795, 2022, ISSN: 1470-8752. @article{Martin2022,
title = {Placental extracellular vesicles in maternal-fetal communication during pregnancy},
author = {Charlène Martin and Mathilde Bergamelli and Cécile E. Malnou and Gisela D'Angelo},
doi = {10.1042/bst20220734},
issn = {1470-8752},
year = {2022},
date = {2022-12-16},
urldate = {2022-12-16},
journal = {Biochem Soc Trans},
volume = {50},
number = {6},
pages = {1785--1795},
publisher = {Portland Press Ltd.},
abstract = {<jats:p>For several years, a growing number of studies have highlighted the pivotal role of placental extracellular vesicles (EVs) throughout pregnancy. These membrane nanovesicles, heterogeneous in nature, composition and origin, are secreted by several trophoblastic cell types and are found in both the maternal and fetal compartments. They can be uptaken by recipient cells and drive a wide variety of physiological and pathological processes. In this review, we provide an overview of the different described roles of placental EVs in various aspects of normal pregnancy, from placenta establishment to maternal immune tolerance towards the fetus and protection against viral infections. In the second part, we present selected examples of pathological pregnancies in which placental EVs are involved, such as gestational diabetes mellitus, pre-eclampsia, and congenital infections. Since the abundance and/or composition of placental EVs is deregulated in maternal serum during pathological pregnancies, this makes them interesting candidates as non-invasive biomarkers for gestational diseases and opens a wide field of translational perspectives.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>For several years, a growing number of studies have highlighted the pivotal role of placental extracellular vesicles (EVs) throughout pregnancy. These membrane nanovesicles, heterogeneous in nature, composition and origin, are secreted by several trophoblastic cell types and are found in both the maternal and fetal compartments. They can be uptaken by recipient cells and drive a wide variety of physiological and pathological processes. In this review, we provide an overview of the different described roles of placental EVs in various aspects of normal pregnancy, from placenta establishment to maternal immune tolerance towards the fetus and protection against viral infections. In the second part, we present selected examples of pathological pregnancies in which placental EVs are involved, such as gestational diabetes mellitus, pre-eclampsia, and congenital infections. Since the abundance and/or composition of placental EVs is deregulated in maternal serum during pathological pregnancies, this makes them interesting candidates as non-invasive biomarkers for gestational diseases and opens a wide field of translational perspectives.</jats:p> |
Tarbouriech, Nicolas; Chenavier, Florian; Kawasaki, Junna; Bachiri, Kamel; Bourhis, Jean-Marie; Legrand, Pierre; Freslon, Lily L.; Laurent, Estelle M. N.; Suberbielle, Elsa; Ruigrok, Rob W. H.; Tomonaga, Keizo; Gonzalez-Dunia, Daniel; Horie, Masayuki; Coyaud, Etienne; Crépin, Thibaut Borna Disease Virus 1 Phosphoprotein Forms a Tetramer and Interacts with Host Factors Involved in DNA Double-Strand Break Repair and mRNA Processing Article de journal Dans: Viruses, vol. 14, no. 11, 2022, ISSN: 1999-4915. @article{Tarbouriech2022,
title = {Borna Disease Virus 1 Phosphoprotein Forms a Tetramer and Interacts with Host Factors Involved in DNA Double-Strand Break Repair and mRNA Processing},
author = {Nicolas Tarbouriech and Florian Chenavier and Junna Kawasaki and Kamel Bachiri and Jean-Marie Bourhis and Pierre Legrand and Lily L. Freslon and Estelle M. N. Laurent and Elsa Suberbielle and Rob W. H. Ruigrok and Keizo Tomonaga and Daniel Gonzalez-Dunia and Masayuki Horie and Etienne Coyaud and Thibaut Crépin},
doi = {10.3390/v14112358},
issn = {1999-4915},
year = {2022},
date = {2022-11-00},
urldate = {2022-11-00},
journal = {Viruses},
volume = {14},
number = {11},
publisher = {MDPI AG},
abstract = {<jats:p>Determining the structural organisation of viral replication complexes and unravelling the impact of infection on cellular homeostasis represent important challenges in virology. This may prove particularly useful when confronted with viruses that pose a significant threat to human health, that appear unique within their family, or for which knowledge is scarce. Among Mononegavirales, bornaviruses (family Bornaviridae) stand out due to their compact genomes and their nuclear localisation for replication. The recent recognition of the zoonotic potential of several orthobornaviruses has sparked a surge of interest in improving our knowledge on this viral family. In this work, we provide a complete analysis of the structural organisation of Borna disease virus 1 (BoDV-1) phosphoprotein (P), an important cofactor for polymerase activity. Using X-ray diffusion and diffraction experiments, we revealed that BoDV-1 P adopts a long coiled-coil α-helical structure split into two parts by an original β-strand twist motif, which is highly conserved across the members of whole Orthobornavirus genus and may regulate viral replication. In parallel, we used BioID to determine the proximal interactome of P in living cells. We confirmed previously known interactors and identified novel proteins linked to several biological processes such as DNA repair or mRNA metabolism. Altogether, our study provides important structure/function cues, which may improve our understanding of BoDV-1 pathogenesis.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>Determining the structural organisation of viral replication complexes and unravelling the impact of infection on cellular homeostasis represent important challenges in virology. This may prove particularly useful when confronted with viruses that pose a significant threat to human health, that appear unique within their family, or for which knowledge is scarce. Among Mononegavirales, bornaviruses (family Bornaviridae) stand out due to their compact genomes and their nuclear localisation for replication. The recent recognition of the zoonotic potential of several orthobornaviruses has sparked a surge of interest in improving our knowledge on this viral family. In this work, we provide a complete analysis of the structural organisation of Borna disease virus 1 (BoDV-1) phosphoprotein (P), an important cofactor for polymerase activity. Using X-ray diffusion and diffraction experiments, we revealed that BoDV-1 P adopts a long coiled-coil α-helical structure split into two parts by an original β-strand twist motif, which is highly conserved across the members of whole Orthobornavirus genus and may regulate viral replication. In parallel, we used BioID to determine the proximal interactome of P in living cells. We confirmed previously known interactors and identified novel proteins linked to several biological processes such as DNA repair or mRNA metabolism. Altogether, our study provides important structure/function cues, which may improve our understanding of BoDV-1 pathogenesis.</jats:p> |
Bergamelli, Mathilde; Martin, Hélène; Aubert, Yann; Mansuy, Jean-Michel; Marcellin, Marlène; Burlet-Schiltz, Odile; Hurbain, Ilse; Raposo, Graça; Izopet, Jacques; Fournier, Thierry; Benchoua, Alexandra; Bénard, Mélinda; Groussolles, Marion; Cartron, Géraldine; Gac, Yann Tanguy Le; Moinard, Nathalie; D’Angelo, Gisela; Malnou, Cécile E. Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro Article de journal Dans: Viruses, vol. 14, no. 9, 2022, ISSN: 1999-4915. @article{Bergamelli2022,
title = {Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro},
author = {Mathilde Bergamelli and Hélène Martin and Yann Aubert and Jean-Michel Mansuy and Marlène Marcellin and Odile Burlet-Schiltz and Ilse Hurbain and Graça Raposo and Jacques Izopet and Thierry Fournier and Alexandra Benchoua and Mélinda Bénard and Marion Groussolles and Géraldine Cartron and Yann Tanguy Le Gac and Nathalie Moinard and Gisela D’Angelo and Cécile E. Malnou},
doi = {10.3390/v14092030},
issn = {1999-4915},
year = {2022},
date = {2022-09-00},
urldate = {2022-09-00},
journal = {Viruses},
volume = {14},
number = {9},
publisher = {MDPI AG},
abstract = {<jats:p>Although placental small extracellular vesicles (sEVs) are extensively studied in the context of pregnancy, little is known about their role during viral congenital infection, especially at the beginning of pregnancy. In this study, we examined the consequences of human cytomegalovirus (hCMV) infection on sEVs production, composition, and function using an immortalized human cytotrophoblast cell line derived from first trimester placenta. By combining complementary approaches of biochemistry, electron microscopy, and quantitative proteomic analysis, we showed that hCMV infection increases the yield of sEVs produced by cytotrophoblasts and modifies their protein content towards a potential proviral phenotype. We further demonstrate that sEVs secreted by hCMV-infected cytotrophoblasts potentiate infection in naive recipient cells of fetal origin, including human neural stem cells. Importantly, these functional consequences are also observed with sEVs prepared from an ex vivo model of infected histocultures from early placenta. Based on these findings, we propose that placental sEVs could be important actors favoring viral dissemination to the fetal brain during hCMV congenital infection.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>Although placental small extracellular vesicles (sEVs) are extensively studied in the context of pregnancy, little is known about their role during viral congenital infection, especially at the beginning of pregnancy. In this study, we examined the consequences of human cytomegalovirus (hCMV) infection on sEVs production, composition, and function using an immortalized human cytotrophoblast cell line derived from first trimester placenta. By combining complementary approaches of biochemistry, electron microscopy, and quantitative proteomic analysis, we showed that hCMV infection increases the yield of sEVs produced by cytotrophoblasts and modifies their protein content towards a potential proviral phenotype. We further demonstrate that sEVs secreted by hCMV-infected cytotrophoblasts potentiate infection in naive recipient cells of fetal origin, including human neural stem cells. Importantly, these functional consequences are also observed with sEVs prepared from an ex vivo model of infected histocultures from early placenta. Based on these findings, we propose that placental sEVs could be important actors favoring viral dissemination to the fetal brain during hCMV congenital infection.</jats:p> |
Martin, Hélène; Barthelemy, Jonathan; Chin, Yamileth; Bergamelli, Mathilde; Moinard, Nathalie; Cartron, Géraldine; Gac, Yann Tanguy Le; Malnou, Cécile E.; Simonin, Yannick Usutu Virus Infects Human Placental Explants and Induces Congenital Defects in Mice Article de journal Dans: Viruses, vol. 14, no. 8, 2022, ISSN: 1999-4915. @article{Martin2022b,
title = {Usutu Virus Infects Human Placental Explants and Induces Congenital Defects in Mice},
author = {Hélène Martin and Jonathan Barthelemy and Yamileth Chin and Mathilde Bergamelli and Nathalie Moinard and Géraldine Cartron and Yann Tanguy Le Gac and Cécile E. Malnou and Yannick Simonin},
doi = {10.3390/v14081619},
issn = {1999-4915},
year = {2022},
date = {2022-08-00},
urldate = {2022-08-00},
journal = {Viruses},
volume = {14},
number = {8},
publisher = {MDPI AG},
abstract = {<jats:p>Usutu virus (USUV) is a neurotropic mosquito-borne flavivirus that has dispersed quickly in Europe these past years. This arbovirus mainly follows an enzootic cycle involving mosquitoes and birds, but can also infect other mammals, causing notably sporadic cases in humans. Although it is mainly asymptomatic or responsible for mild clinical symptoms, USUV has been associated with neurological disorders, such as encephalitis and meningoencephalitis, highlighting the potential health threat of this virus. Among the different transmission routes described for other flaviviruses, the capacity for some of them to be transmitted vertically has been demonstrated, notably for Zika virus or West Nile virus, which are closely related to USUV. To evaluate the ability of USUV to replicate in the placenta and gain access to the fetus, we combined the use of several trophoblast model cell lines, ex vivo human placental explant cultures from first and third trimester of pregnancy, and in vivo USUV-infected pregnant mice. Our data demonstrate that human placental cells and tissues are permissive to USUV replication, and suggest that viral transmission can occur in mice during gestation. Hence, our observations suggest that USUV could be efficiently transmitted by the vertical route.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>Usutu virus (USUV) is a neurotropic mosquito-borne flavivirus that has dispersed quickly in Europe these past years. This arbovirus mainly follows an enzootic cycle involving mosquitoes and birds, but can also infect other mammals, causing notably sporadic cases in humans. Although it is mainly asymptomatic or responsible for mild clinical symptoms, USUV has been associated with neurological disorders, such as encephalitis and meningoencephalitis, highlighting the potential health threat of this virus. Among the different transmission routes described for other flaviviruses, the capacity for some of them to be transmitted vertically has been demonstrated, notably for Zika virus or West Nile virus, which are closely related to USUV. To evaluate the ability of USUV to replicate in the placenta and gain access to the fetus, we combined the use of several trophoblast model cell lines, ex vivo human placental explant cultures from first and third trimester of pregnancy, and in vivo USUV-infected pregnant mice. Our data demonstrate that human placental cells and tissues are permissive to USUV replication, and suggest that viral transmission can occur in mice during gestation. Hence, our observations suggest that USUV could be efficiently transmitted by the vertical route.</jats:p> |
Quelle est la réalité des infections humaines par les Bornavirus ? Article de journal Dans: Virologie, vol. 26, no. 4, p. 275–281, 2022, ISSN: 1267-8694. @article{2022,
title = {Quelle est la réalité des infections humaines par les Bornavirus ?},
doi = {10.1684/vir.2022.0965},
issn = {1267-8694},
year = {2022},
date = {2022-07-01},
urldate = {2022-07-01},
journal = {Virologie},
volume = {26},
number = {4},
pages = {275--281},
publisher = {John Libbey Eurotext},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Marty, Florent Henri; Bettamin, Luca; Thouard, Anne; Bourgade, Karine; Allart, Sophie; Larrieu, Guilhem; Malnou, Cécile Evelyne; Gonzalez-Dunia, Daniel; Suberbielle, Elsa Borna disease virus docks on neuronal DNA double-strand breaks to replicate and dampens neuronal activity Article de journal Dans: iScience, vol. 25, no. 1, 2022, ISSN: 2589-0042. @article{Marty2022,
title = {Borna disease virus docks on neuronal DNA double-strand breaks to replicate and dampens neuronal activity},
author = {Florent Henri Marty and Luca Bettamin and Anne Thouard and Karine Bourgade and Sophie Allart and Guilhem Larrieu and Cécile Evelyne Malnou and Daniel Gonzalez-Dunia and Elsa Suberbielle},
doi = {10.1016/j.isci.2021.103621},
issn = {2589-0042},
year = {2022},
date = {2022-01-00},
urldate = {2022-01-00},
journal = {iScience},
volume = {25},
number = {1},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Ligat, Gaëtan; Ghelfenstein-Ferreira, Théo; Dellière, Sarah; Pichon, Maxime Bringing clinical and fundamental young microbiologists together Article de journal Dans: FEMS Microbes, vol. 3, 2022, ISSN: 2633-6685. @article{Ligat2022,
title = {Bringing clinical and fundamental young microbiologists together},
author = {Gaëtan Ligat and Théo Ghelfenstein-Ferreira and Sarah Dellière and Maxime Pichon},
doi = {10.1093/femsmc/xtac025},
issn = {2633-6685},
year = {2022},
date = {2022-00-00},
urldate = {2022-00-00},
journal = {FEMS Microbes},
volume = {3},
publisher = {Oxford University Press (OUP)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2021
|
Ferré, Cécile A.; Thouard, Anne; Bétourné, Alexandre; Dorze, Anne-Louise Le; Belenguer, Pascale; Miquel, Marie-Christine; Peyrin, Jean-Michel; Gonzalez-Dunia, Daniel; Szelechowski, Marion HSPA9/Mortalin mediates axo-protection and modulates mitochondrial dynamics in neurons Article de journal Dans: Sci Rep, vol. 11, no. 1, 2021, ISSN: 2045-2322. @article{Ferré2021,
title = {HSPA9/Mortalin mediates axo-protection and modulates mitochondrial dynamics in neurons},
author = {Cécile A. Ferré and Anne Thouard and Alexandre Bétourné and Anne-Louise Le Dorze and Pascale Belenguer and Marie-Christine Miquel and Jean-Michel Peyrin and Daniel Gonzalez-Dunia and Marion Szelechowski},
doi = {10.1038/s41598-021-97162-1},
issn = {2045-2322},
year = {2021},
date = {2021-12-00},
urldate = {2021-12-00},
journal = {Sci Rep},
volume = {11},
number = {1},
publisher = {Springer Science and Business Media LLC},
abstract = {<jats:title>Abstract</jats:title><jats:p>Mortalin is a mitochondrial chaperone protein involved in quality control of proteins imported into the mitochondrial matrix, which was recently described as a sensor of neuronal stress. Mortalin is down-regulated in neurons of patients with neurodegenerative diseases and levels of Mortalin expression are correlated with neuronal fate in animal models of Alzheimer's disease or cerebral ischemia. To date, however, the links between Mortalin levels, its impact on mitochondrial function and morphology and, ultimately, the initiation of neurodegeneration, are still unclear. In the present study, we used lentiviral vectors to over- or under-express Mortalin in primary neuronal cultures. We first analyzed the early events of neurodegeneration in the axonal compartment, using oriented neuronal cultures grown in microfluidic-based devices. We observed that Mortalin down-regulation induced mitochondrial fragmentation and axonal damage, whereas its over-expression conferred protection against axonal degeneration mediated by rotenone exposure. We next demonstrated that Mortalin levels modulated mitochondrial morphology by acting on DRP1 phosphorylation, thereby further illustrating the crucial implication of mitochondrial dynamics on neuronal fate in degenerative diseases.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Mortalin is a mitochondrial chaperone protein involved in quality control of proteins imported into the mitochondrial matrix, which was recently described as a sensor of neuronal stress. Mortalin is down-regulated in neurons of patients with neurodegenerative diseases and levels of Mortalin expression are correlated with neuronal fate in animal models of Alzheimer's disease or cerebral ischemia. To date, however, the links between Mortalin levels, its impact on mitochondrial function and morphology and, ultimately, the initiation of neurodegeneration, are still unclear. In the present study, we used lentiviral vectors to over- or under-express Mortalin in primary neuronal cultures. We first analyzed the early events of neurodegeneration in the axonal compartment, using oriented neuronal cultures grown in microfluidic-based devices. We observed that Mortalin down-regulation induced mitochondrial fragmentation and axonal damage, whereas its over-expression conferred protection against axonal degeneration mediated by rotenone exposure. We next demonstrated that Mortalin levels modulated mitochondrial morphology by acting on DRP1 phosphorylation, thereby further illustrating the crucial implication of mitochondrial dynamics on neuronal fate in degenerative diseases.</jats:p> |
Bourgade, Karine; Thouard, Anne; Abravanel, Florence; Hebral, Anne‐Laure; Bello, Arnaud Del; Viguier, Alain; Gonzalez‐Dunia, Daniel; Kamar, Nassim Fatal encephalitis and Borna Disease Virus‐1 seropositivity in two kidney‐transplant patients living in the same nonendemic area Article de journal Dans: Transplant Infectious Dis, vol. 23, no. 6, 2021, ISSN: 1399-3062. @article{Bourgade2021,
title = {Fatal encephalitis and Borna Disease Virus‐1 seropositivity in two kidney‐transplant patients living in the same nonendemic area},
author = {Karine Bourgade and Anne Thouard and Florence Abravanel and Anne‐Laure Hebral and Arnaud Del Bello and Alain Viguier and Daniel Gonzalez‐Dunia and Nassim Kamar},
doi = {10.1111/tid.13734},
issn = {1399-3062},
year = {2021},
date = {2021-12-00},
urldate = {2021-12-00},
journal = {Transplant Infectious Dis},
volume = {23},
number = {6},
publisher = {Wiley},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bergamelli, Mathilde; Martin, Hélène; Bénard, Mélinda; Ausseil, Jérôme; Mansuy, Jean-Michel; Hurbain, Ilse; Mouysset, Maïlys; Groussolles, Marion; Cartron, Géraldine; le Gac, Yann Tanguy; Moinard, Nathalie; Suberbielle, Elsa; Izopet, Jacques; Tscherning, Charlotte; Raposo, Graça; Gonzalez-Dunia, Daniel; D’Angelo, Gisela; Malnou, Cécile E. Human Cytomegalovirus Infection Changes the Pattern of Surface Markers of Small Extracellular Vesicles Isolated From First Trimester Placental Long-Term Histocultures Article de journal Dans: Front. Cell Dev. Biol., vol. 9, 2021, ISSN: 2296-634X. @article{Bergamelli2021,
title = {Human Cytomegalovirus Infection Changes the Pattern of Surface Markers of Small Extracellular Vesicles Isolated From First Trimester Placental Long-Term Histocultures},
author = {Mathilde Bergamelli and Hélène Martin and Mélinda Bénard and Jérôme Ausseil and Jean-Michel Mansuy and Ilse Hurbain and Maïlys Mouysset and Marion Groussolles and Géraldine Cartron and Yann Tanguy le Gac and Nathalie Moinard and Elsa Suberbielle and Jacques Izopet and Charlotte Tscherning and Graça Raposo and Daniel Gonzalez-Dunia and Gisela D’Angelo and Cécile E. Malnou},
doi = {10.3389/fcell.2021.689122},
issn = {2296-634X},
year = {2021},
date = {2021-09-10},
urldate = {2021-09-10},
journal = {Front. Cell Dev. Biol.},
volume = {9},
publisher = {Frontiers Media SA},
abstract = {<jats:p>Extracellular vesicles (EVs) have increasingly been recognized as key players in a wide variety of physiological and pathological contexts, including during pregnancy. Notably, EVs appear both as possible biomarkers and as mediators involved in the communication of the placenta with the maternal and fetal sides. A better understanding of the physiological and pathological roles of EVs strongly depends on the development of adequate and reliable study models, specifically at the beginning of pregnancy where many adverse pregnancy outcomes have their origin. In this study, we describe the isolation of small EVs from a histoculture model of first trimester placental explants in normal conditions as well as upon infection by human cytomegalovirus. Using bead-based multiplex cytometry and electron microscopy combined with biochemical approaches, we characterized these small EVs and defined their associated markers and ultrastructure. We observed that infection led to changes in the expression level of several surface markers, without affecting the secretion and integrity of small EVs. Our findings lay the foundation for studying the functional role of EVs during early pregnancy, along with the identification of new predictive biomarkers for the severity and outcome of this congenital infection, which are still sorely lacking.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:p>Extracellular vesicles (EVs) have increasingly been recognized as key players in a wide variety of physiological and pathological contexts, including during pregnancy. Notably, EVs appear both as possible biomarkers and as mediators involved in the communication of the placenta with the maternal and fetal sides. A better understanding of the physiological and pathological roles of EVs strongly depends on the development of adequate and reliable study models, specifically at the beginning of pregnancy where many adverse pregnancy outcomes have their origin. In this study, we describe the isolation of small EVs from a histoculture model of first trimester placental explants in normal conditions as well as upon infection by human cytomegalovirus. Using bead-based multiplex cytometry and electron microscopy combined with biochemical approaches, we characterized these small EVs and defined their associated markers and ultrastructure. We observed that infection led to changes in the expression level of several surface markers, without affecting the secretion and integrity of small EVs. Our findings lay the foundation for studying the functional role of EVs during early pregnancy, along with the identification of new predictive biomarkers for the severity and outcome of this congenital infection, which are still sorely lacking.</jats:p> |
Rolland, Maude; Martin, Hélène; Bergamelli, Mathilde; Sellier, Yann; Bessières, Bettina; Aziza, Jacqueline; Benchoua, Alexandra; Leruez‐Ville, Marianne; Gonzalez‐Dunia, Daniel; Chavanas, Stéphane Human cytomegalovirus infection is associated with increased expression of the lissencephaly gene <scp> PAFAH1B1 </scp> encoding <scp>LIS1</scp> in neural stem cells and congenitally infected brains Article de journal Dans: J. Pathol., 2021, ISSN: 1096-9896. @article{Rolland2021,
title = {Human cytomegalovirus infection is associated with increased expression of the lissencephaly gene <scp> \textit{PAFAH1B1} </scp> encoding <scp>LIS1</scp> in neural stem cells and congenitally infected brains},
author = {Maude Rolland and Hélène Martin and Mathilde Bergamelli and Yann Sellier and Bettina Bessières and Jacqueline Aziza and Alexandra Benchoua and Marianne Leruez‐Ville and Daniel Gonzalez‐Dunia and Stéphane Chavanas},
doi = {10.1002/path.5640},
issn = {1096-9896},
year = {2021},
date = {2021-03-24},
urldate = {2021-03-24},
journal = {J. Pathol.},
publisher = {Wiley},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2020
|
Portal, Benjamin; Delcourte, Sarah; Rovera, Renaud; Lejards, Camille; Bullich, Sebastien; Malnou, Cécile E.; Haddjeri, Nasser; Déglon, Nicole; Guiard, Bruno P. Genetic and pharmacological inactivation of astroglial connexin 43 differentially influences the acute response of antidepressant and anxiolytic drugs Article de journal Dans: Acta Physiologica, vol. 229, no. 1, 2020, ISSN: 1748-1716. @article{Portal2020,
title = {Genetic and pharmacological inactivation of astroglial connexin 43 differentially influences the acute response of antidepressant and anxiolytic drugs},
author = {Benjamin Portal and Sarah Delcourte and Renaud Rovera and Camille Lejards and Sebastien Bullich and Cécile E. Malnou and Nasser Haddjeri and Nicole Déglon and Bruno P. Guiard},
doi = {10.1111/apha.13440},
issn = {1748-1716},
year = {2020},
date = {2020-05-00},
urldate = {2020-05-00},
journal = {Acta Physiologica},
volume = {229},
number = {1},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:sec><jats:title>Aim</jats:title><jats:p>Astroglial connexins (Cxs) 30 and 43 are engaged in gap junction and hemichannel activities. Evidence suggests that these functional entities contribute to regulating neurotransmission, thereby influencing brain functions. In particular, preclinical and clinical findings highlight a role of Cx43 in animal models of depression. However, the role of these proteins in response to currently available psychotropic drugs is still unknown.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>To investigate this, we evaluated the behavioural effects of the genetic and pharmacological inactivation of Cx43 on the antidepressant‐ and anxiolytic‐like activities of the selective serotonin reuptake inhibitor fluoxetine and the benzodiazepine diazepam, respectively.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A single administration of fluoxetine (18 mg/kg; i.p.) produced a higher increase in hippocampal extracellular serotonin levels, and a greater antidepressant‐like effect in the tail suspension test in Cx43 knock‐down (KD) mice bred on a C57BL/6 background compared to their wild‐type littermates. Similarly, in outbred Swiss wild‐type mice, the intra‐hippocampal injection of a shRNA‐Cx43 or the acute systemic injection of the Cxs inhibitor carbenoxolone (CBX: 10 mg/kg; i.p.) potentiated the antidepressant‐like effects of fluoxetine. Evaluating the effects of such strategies on diazepam (0.5 mg/kg; i.p.), the results indicate that Cx43 KD mice or wild‐types injected with a shRNA‐Cx43 in the amygdala, but not in the hippocampus, attenuated the anxiolytic‐like effects of this benzodiazepine in the elevated plus maze. The chronic systemic administration of CBX mimicked the latter observations.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Collectively, these data pave the way to the development of potentiating strategies in the field of psychiatry based on the modulation of astroglial Cx43.</jats:p></jats:sec>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:sec><jats:title>Aim</jats:title><jats:p>Astroglial connexins (Cxs) 30 and 43 are engaged in gap junction and hemichannel activities. Evidence suggests that these functional entities contribute to regulating neurotransmission, thereby influencing brain functions. In particular, preclinical and clinical findings highlight a role of Cx43 in animal models of depression. However, the role of these proteins in response to currently available psychotropic drugs is still unknown.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>To investigate this, we evaluated the behavioural effects of the genetic and pharmacological inactivation of Cx43 on the antidepressant‐ and anxiolytic‐like activities of the selective serotonin reuptake inhibitor fluoxetine and the benzodiazepine diazepam, respectively.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A single administration of fluoxetine (18 mg/kg; i.p.) produced a higher increase in hippocampal extracellular serotonin levels, and a greater antidepressant‐like effect in the tail suspension test in Cx43 knock‐down (KD) mice bred on a C57BL/6 background compared to their wild‐type littermates. Similarly, in outbred Swiss wild‐type mice, the intra‐hippocampal injection of a shRNA‐Cx43 or the acute systemic injection of the Cxs inhibitor carbenoxolone (CBX: 10 mg/kg; i.p.) potentiated the antidepressant‐like effects of fluoxetine. Evaluating the effects of such strategies on diazepam (0.5 mg/kg; i.p.), the results indicate that Cx43 KD mice or wild‐types injected with a shRNA‐Cx43 in the amygdala, but not in the hippocampus, attenuated the anxiolytic‐like effects of this benzodiazepine in the elevated plus maze. The chronic systemic administration of CBX mimicked the latter observations.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Collectively, these data pave the way to the development of potentiating strategies in the field of psychiatry based on the modulation of astroglial Cx43.</jats:p></jats:sec> |
2019
|
de Paz, Alexia Martínez; Khajavi, Leila; Martin, Hélène; Claveria-Gimeno, Rafael; Dieck, Susanne Tom; Cheema, Manjinder S.; Sanchez-Mut, Jose V.; Moksa, Malgorzata M.; Carles, Annaick; Brodie, Nick I.; Sheikh, Taimoor I.; Freeman, Melissa E.; Petrotchenko, Evgeniy V.; Borchers, Christoph H.; Schuman, Erin M.; Zytnicki, Matthias; Velazquez-Campoy, Adrian; Abian, Olga; Hirst, Martin; Esteller, Manel; Vincent, John B.; Malnou, Cécile E.; Ausió, Juan MeCP2-E1 isoform is a dynamically expressed, weakly DNA-bound protein with different protein and DNA interactions compared to MeCP2-E2 Article de journal Dans: Epigenetics & Chromatin, vol. 12, no. 1, 2019, ISSN: 1756-8935. @article{MartínezdePaz2019,
title = {MeCP2-E1 isoform is a dynamically expressed, weakly DNA-bound protein with different protein and DNA interactions compared to MeCP2-E2},
author = {Alexia Martínez de Paz and Leila Khajavi and Hélène Martin and Rafael Claveria-Gimeno and Susanne Tom Dieck and Manjinder S. Cheema and Jose V. Sanchez-Mut and Malgorzata M. Moksa and Annaick Carles and Nick I. Brodie and Taimoor I. Sheikh and Melissa E. Freeman and Evgeniy V. Petrotchenko and Christoph H. Borchers and Erin M. Schuman and Matthias Zytnicki and Adrian Velazquez-Campoy and Olga Abian and Martin Hirst and Manel Esteller and John B. Vincent and Cécile E. Malnou and Juan Ausió},
doi = {10.1186/s13072-019-0298-1},
issn = {1756-8935},
year = {2019},
date = {2019-12-00},
urldate = {2019-12-00},
journal = {Epigenetics & Chromatin},
volume = {12},
number = {1},
publisher = {Springer Science and Business Media LLC},
abstract = {<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>MeCP2—a chromatin-binding protein associated with Rett syndrome—has two main isoforms, MeCP2-E1 and MeCP2-E2, differing in a few N-terminal amino acid residues. Previous studies have shown brain region-specific expression of these isoforms which, in addition to their different cellular localization and differential expression during brain development, suggest that they may also have non-overlapping molecular mechanisms. However, differential functions of MeCP2-E1 and E2 remain largely unexplored.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Here, we show that the N-terminal domains (NTD) of MeCP2-E1 and E2 modulate the ability of the methyl-binding domain (MBD) to interact with DNA as well as influencing the turn-over rates, binding dynamics, response to neuronal depolarization, and circadian oscillations of the two isoforms. Our proteomics data indicate that both isoforms exhibit unique interacting protein partners. Moreover, genome-wide analysis using ChIP-seq provide evidence for a shared as well as a specific regulation of different sets of genes.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Our study supports the idea that Rett syndrome might arise from simultaneous impairment of cellular processes involving non-overlapping functions of MECP2 isoforms. For instance, MeCP2-E1 mutations might impact stimuli-dependent chromatin regulation, while MeCP2-E2 mutations could result in aberrant ribosomal expression. Overall, our findings provide insight into the functional complexity of MeCP2 by dissecting differential aspects of its two isoforms.</jats:p></jats:sec>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>MeCP2—a chromatin-binding protein associated with Rett syndrome—has two main isoforms, MeCP2-E1 and MeCP2-E2, differing in a few N-terminal amino acid residues. Previous studies have shown brain region-specific expression of these isoforms which, in addition to their different cellular localization and differential expression during brain development, suggest that they may also have non-overlapping molecular mechanisms. However, differential functions of MeCP2-E1 and E2 remain largely unexplored.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Here, we show that the N-terminal domains (NTD) of MeCP2-E1 and E2 modulate the ability of the methyl-binding domain (MBD) to interact with DNA as well as influencing the turn-over rates, binding dynamics, response to neuronal depolarization, and circadian oscillations of the two isoforms. Our proteomics data indicate that both isoforms exhibit unique interacting protein partners. Moreover, genome-wide analysis using ChIP-seq provide evidence for a shared as well as a specific regulation of different sets of genes.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Our study supports the idea that Rett syndrome might arise from simultaneous impairment of cellular processes involving non-overlapping functions of MECP2 isoforms. For instance, MeCP2-E1 mutations might impact stimuli-dependent chromatin regulation, while MeCP2-E2 mutations could result in aberrant ribosomal expression. Overall, our findings provide insight into the functional complexity of MeCP2 by dissecting differential aspects of its two isoforms.</jats:p></jats:sec> |
Malnou, E. Cécile; Umlauf, David; Mouysset, Maïlys; Cavaillé, Jérôme Imprinted MicroRNA Gene Clusters in the Evolution, Development, and Functions of Mammalian Placenta Article de journal Dans: Front. Genet., vol. 9, 2019, ISSN: 1664-8021. @article{Malnou2019,
title = {Imprinted MicroRNA Gene Clusters in the Evolution, Development, and Functions of Mammalian Placenta},
author = {E. Cécile Malnou and David Umlauf and Maïlys Mouysset and Jérôme Cavaillé},
doi = {10.3389/fgene.2018.00706},
issn = {1664-8021},
year = {2019},
date = {2019-01-18},
urldate = {2019-01-18},
journal = {Front. Genet.},
volume = {9},
publisher = {Frontiers Media SA},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2018
|
Casanova, A; Bettamin, L; Blatche, M-C; Mathieu, F; Martin, H; Gonzalez-Dunia, D; Nicu, L; Larrieu, G Nanowire based bioprobes for electrical monitoring of electrogenic cells Article de journal Dans: J. Phys.: Condens. Matter, vol. 30, no. 46, 2018, ISSN: 1361-648X. @article{Casanova2018,
title = {Nanowire based bioprobes for electrical monitoring of electrogenic cells},
author = {A Casanova and L Bettamin and M-C Blatche and F Mathieu and H Martin and D Gonzalez-Dunia and L Nicu and G Larrieu},
doi = {10.1088/1361-648x/aae5aa},
issn = {1361-648X},
year = {2018},
date = {2018-11-21},
urldate = {2018-11-21},
journal = {J. Phys.: Condens. Matter},
volume = {30},
number = {46},
publisher = {IOP Publishing},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bétourné, Alexandre; Szelechowski, Marion; Thouard, Anne; Abrial, Erika; Jean, Arnaud; Zaidi, Falek; Foret, Charlotte; Bonnaud, Emilie M.; Charlier, Caroline M.; Suberbielle, Elsa; Malnou, Cécile E.; Granon, Sylvie; Rampon, Claire; Gonzalez-Dunia, Daniel Hippocampal expression of a virus-derived protein impairs memory in mice Article de journal Dans: Proc. Natl. Acad. Sci. U.S.A., vol. 115, no. 7, p. 1611–1616, 2018, ISSN: 1091-6490. @article{Bétourné2018,
title = {Hippocampal expression of a virus-derived protein impairs memory in mice},
author = {Alexandre Bétourné and Marion Szelechowski and Anne Thouard and Erika Abrial and Arnaud Jean and Falek Zaidi and Charlotte Foret and Emilie M. Bonnaud and Caroline M. Charlier and Elsa Suberbielle and Cécile E. Malnou and Sylvie Granon and Claire Rampon and Daniel Gonzalez-Dunia},
doi = {10.1073/pnas.1711977115},
issn = {1091-6490},
year = {2018},
date = {2018-02-13},
urldate = {2018-02-13},
journal = {Proc. Natl. Acad. Sci. U.S.A.},
volume = {115},
number = {7},
pages = {1611--1616},
publisher = {Proceedings of the National Academy of Sciences},
abstract = {<jats:title>Significance</jats:title>
<jats:p>As obligate parasites, viruses have evolved strategies to hijack cellular pathways and persist in their host, sometimes without causing overt diseases. As such, they represent unique tools to decipher cellular functions and their consequences on host physiology. Here, we exploited the natural property of a virus-encoded protein known to act as a decoy substrate for protein kinase C (PKC), a pathway thought to play key roles in learning and memory processes. When selectively expressed in the hippocampal dentate gyrus of mice, this protein caused behavioral abnormalities, notably increased anxiety and impaired memory, mostly by interfering with PKC-dependent phosphorylation. Our findings provide further insight into the role of the PKC pathway in controlling cognitive functions.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Significance</jats:title>
<jats:p>As obligate parasites, viruses have evolved strategies to hijack cellular pathways and persist in their host, sometimes without causing overt diseases. As such, they represent unique tools to decipher cellular functions and their consequences on host physiology. Here, we exploited the natural property of a virus-encoded protein known to act as a decoy substrate for protein kinase C (PKC), a pathway thought to play key roles in learning and memory processes. When selectively expressed in the hippocampal dentate gyrus of mice, this protein caused behavioral abnormalities, notably increased anxiety and impaired memory, mostly by interfering with PKC-dependent phosphorylation. Our findings provide further insight into the role of the PKC pathway in controlling cognitive functions.</jats:p> |
2017
|
Denadai-Souza, Alexandre; Ribeiro, Camilla Moreira; Rolland, Corinne; Thouard, Anne; Deraison, Céline; Scavone, Cristoforo; Gonzalez-Dunia, Daniel; Vergnolle, Nathalie; Avellar, Maria Christina Werneck Effect of tryptase inhibition on joint inflammation: a pharmacological and lentivirus-mediated gene transfer study Article de journal Dans: Arthritis Res Ther, vol. 19, no. 1, 2017, ISSN: 1478-6362. @article{Denadai-Souza2017,
title = {Effect of tryptase inhibition on joint inflammation: a pharmacological and lentivirus-mediated gene transfer study},
author = {Alexandre Denadai-Souza and Camilla Moreira Ribeiro and Corinne Rolland and Anne Thouard and Céline Deraison and Cristoforo Scavone and Daniel Gonzalez-Dunia and Nathalie Vergnolle and Maria Christina Werneck Avellar},
doi = {10.1186/s13075-017-1326-9},
issn = {1478-6362},
year = {2017},
date = {2017-12-00},
urldate = {2017-12-00},
journal = {Arthritis Res Ther},
volume = {19},
number = {1},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2016
|
Charlier, Caroline M.; Debaisieux, Solène; Foret, Charlotte; Thouard, Anne; Schiavo, Giampietro; Gonzalez-Dunia, Daniel; Malnou, Cécile E. Neuronal retrograde transport of Borna disease virus occurs in signalling endosomes Article de journal Dans: J Gen Virol, vol. 97, no. 12, p. 3215–3224, 2016, ISSN: 1465-2099. @article{Charlier2016,
title = {Neuronal retrograde transport of Borna disease virus occurs in signalling endosomes},
author = {Caroline M. Charlier and Solène Debaisieux and Charlotte Foret and Anne Thouard and Giampietro Schiavo and Daniel Gonzalez-Dunia and Cécile E. Malnou},
doi = {10.1099/jgv.0.000652},
issn = {1465-2099},
year = {2016},
date = {2016-12-16},
urldate = {2016-12-16},
journal = {J Gen Virol},
volume = {97},
number = {12},
pages = {3215--3224},
publisher = {Microbiology Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Martin, Hélène; Laborel-Préneron, Emeline; Fraysse, Frédérique; Nguyen, Thien; Schmitt, Anne-Marie; Redoulès, Daniel; Davrinche, Christian Aquaphilus dolomiaeextract counteracts the effects of cutaneousS. aureussecretome isolated from atopic children on CD4+T cell activation Article de journal Dans: Pharmaceutical Biology, vol. 54, no. 11, p. 2782–2785, 2016, ISSN: 1744-5116. @article{Martin2016,
title = {\textit{Aquaphilus dolomiae}extract counteracts the effects of cutaneous\textit{S. aureus}secretome isolated from atopic children on CD4^{+}T cell activation},
author = {Hélène Martin and Emeline Laborel-Préneron and Frédérique Fraysse and Thien Nguyen and Anne-Marie Schmitt and Daniel Redoulès and Christian Davrinche},
doi = {10.3109/13880209.2016.1173069},
issn = {1744-5116},
year = {2016},
date = {2016-11-00},
urldate = {2016-11-00},
journal = {Pharmaceutical Biology},
volume = {54},
number = {11},
pages = {2782--2785},
publisher = {Informa UK Limited},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bonnaud, Emilie M.; Suberbielle, Elsa; Malnou, Cécile E. Histone acetylation in neuronal (dys)function Article de journal Dans: Biomolecular Concepts, vol. 7, no. 2, p. 103–116, 2016, ISSN: 1868-503X. @article{Bonnaud2016,
title = {Histone acetylation in neuronal (dys)function},
author = {Emilie M. Bonnaud and Elsa Suberbielle and Cécile E. Malnou},
doi = {10.1515/bmc-2016-0002},
issn = {1868-503X},
year = {2016},
date = {2016-05-01},
urldate = {2016-05-01},
journal = {Biomolecular Concepts},
volume = {7},
number = {2},
pages = {103--116},
publisher = {Walter de Gruyter GmbH},
abstract = {<jats:title>Abstract</jats:title><jats:p>Cognitive functions require the expression of an appropriate pattern of genes in response to environmental stimuli. Over the last years, many studies have accumulated knowledge towards the understanding of molecular mechanisms that regulate neuronal gene expression. Epigenetic modifications have been shown to play an important role in numerous neuronal functions, from synaptic plasticity to learning and memory. In particular, histone acetylation is a central player in these processes. In this review, we present the molecular mechanisms of histone acetylation and summarize the data underlying the relevance of histone acetylation in cognitive functions in normal and pathological conditions. In the last part, we discuss the different mechanisms underlying the dysregulation of histone acetylation associated with neurological disorders, with a particular focus on environmental causes (stress, drugs, or infectious agents) that are linked to impaired histone acetylation.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Cognitive functions require the expression of an appropriate pattern of genes in response to environmental stimuli. Over the last years, many studies have accumulated knowledge towards the understanding of molecular mechanisms that regulate neuronal gene expression. Epigenetic modifications have been shown to play an important role in numerous neuronal functions, from synaptic plasticity to learning and memory. In particular, histone acetylation is a central player in these processes. In this review, we present the molecular mechanisms of histone acetylation and summarize the data underlying the relevance of histone acetylation in cognitive functions in normal and pathological conditions. In the last part, we discuss the different mechanisms underlying the dysregulation of histone acetylation associated with neurological disorders, with a particular focus on environmental causes (stress, drugs, or infectious agents) that are linked to impaired histone acetylation.</jats:p> |
Rolland, Maude; Li, Xiaojun; Sellier, Yann; Martin, Hélène; Perez-Berezo, Teresa; Rauwel, Benjamin; Benchoua, Alexandra; Bessières, Bettina; Aziza, Jacqueline; Cenac, Nicolas; Luo, Minhua; Casper, Charlotte; Peschanski, Marc; Gonzalez-Dunia, Daniel; Leruez-Ville, Marianne; Davrinche, Christian; Chavanas, Stéphane PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells Article de journal Dans: PLoS Pathog, vol. 12, no. 4, 2016, ISSN: 1553-7374. @article{Rolland2016,
title = {PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells},
author = {Maude Rolland and Xiaojun Li and Yann Sellier and Hélène Martin and Teresa Perez-Berezo and Benjamin Rauwel and Alexandra Benchoua and Bettina Bessières and Jacqueline Aziza and Nicolas Cenac and Minhua Luo and Charlotte Casper and Marc Peschanski and Daniel Gonzalez-Dunia and Marianne Leruez-Ville and Christian Davrinche and Stéphane Chavanas},
editor = {Robert F. Kalejta},
doi = {10.1371/journal.ppat.1005547},
issn = {1553-7374},
year = {2016},
date = {2016-04-14},
urldate = {2016-04-14},
journal = {PLoS Pathog},
volume = {12},
number = {4},
publisher = {Public Library of Science (PLoS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Ferré, Cécile A.; Davezac, Noelie; Thouard, Anne; Peyrin, Jean‐Michel; Belenguer, Pascale; Miquel, Marie‐Christine; Gonzalez‐Dunia, Daniel; Szelechowski, Marion Manipulation of the N‐terminal sequence of the Borna disease virus X protein improves its mitochondrial targeting and neuroprotective potential Article de journal Dans: FASEB j., vol. 30, no. 4, p. 1523–1533, 2016, ISSN: 1530-6860. @article{Ferré2015,
title = {Manipulation of the N‐terminal sequence of the Borna disease virus X protein improves its mitochondrial targeting and neuroprotective potential},
author = {Cécile A. Ferré and Noelie Davezac and Anne Thouard and Jean‐Michel Peyrin and Pascale Belenguer and Marie‐Christine Miquel and Daniel Gonzalez‐Dunia and Marion Szelechowski},
doi = {10.1096/fj.15-279620},
issn = {1530-6860},
year = {2016},
date = {2016-04-00},
urldate = {2016-04-00},
journal = {FASEB j.},
volume = {30},
number = {4},
pages = {1523--1533},
publisher = {Wiley},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Chavanas, Stéphane Peroxisome proliferator-activated receptor γ (PPARγ) activation: A key determinant of neuropathogeny during congenital infection by cytomegalovirus Article de journal Dans: Neurogenesis, vol. 3, no. 1, 2016, ISSN: 2326-2133. @article{Chavanas2016,
title = {Peroxisome proliferator-activated receptor γ (PPARγ) activation: A key determinant of neuropathogeny during congenital infection by cytomegalovirus},
author = {Stéphane Chavanas},
doi = {10.1080/23262133.2016.1231654},
issn = {2326-2133},
year = {2016},
date = {2016-01-00},
urldate = {2016-01-00},
journal = {Neurogenesis},
volume = {3},
number = {1},
publisher = {Informa UK Limited},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2015
|
Suberbielle, Elsa; Djukic, Biljana; Evans, Mark; Kim, Daniel H.; Taneja, Praveen; Wang, Xin; Finucane, Mariel; Knox, Joseph; Ho, Kaitlyn; Devidze, Nino; Masliah, Eliezer; Mucke, Lennart DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice Article de journal Dans: Nat Commun, vol. 6, no. 1, 2015, ISSN: 2041-1723. @article{Suberbielle2015,
title = {DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice},
author = {Elsa Suberbielle and Biljana Djukic and Mark Evans and Daniel H. Kim and Praveen Taneja and Xin Wang and Mariel Finucane and Joseph Knox and Kaitlyn Ho and Nino Devidze and Eliezer Masliah and Lennart Mucke},
doi = {10.1038/ncomms9897},
issn = {2041-1723},
year = {2015},
date = {2015-12-22},
urldate = {2015-12-22},
journal = {Nat Commun},
volume = {6},
number = {1},
publisher = {Springer Science and Business Media LLC},
abstract = {<jats:title>Abstract</jats:title><jats:p>Maintaining DNA integrity is vital for all cells and organisms. Defective DNA repair may contribute to neurological disorders, including Alzheimer’s disease (AD). We found reduced levels of BRCA1, but not of other DNA repair factors, in the brains of AD patients and human amyloid precursor protein (hAPP) transgenic mice. Amyloid-β oligomers reduced BRCA1 levels in primary neuronal cultures. In wild-type mice, knocking down neuronal BRCA1 in the dentate gyrus caused increased DNA double-strand breaks, neuronal shrinkage, synaptic plasticity impairments, and learning and memory deficits, but not apoptosis. Low levels of hAPP/Amyloid-β overexpression exacerbated these effects. Physiological neuronal activation increased BRCA1 levels, whereas stimulating predominantly extrasynaptic <jats:italic>N</jats:italic>-methyl-<jats:sc>D</jats:sc>-aspartate receptors promoted the proteasomal degradation of BRCA1. We conclude that BRCA1 is regulated by neuronal activity, protects the neuronal genome, and critically supports neuronal integrity and cognitive functions. Pathological accumulation of Aβ depletes neuronal BRCA1, which may contribute to cognitive deficits in AD.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Maintaining DNA integrity is vital for all cells and organisms. Defective DNA repair may contribute to neurological disorders, including Alzheimer’s disease (AD). We found reduced levels of BRCA1, but not of other DNA repair factors, in the brains of AD patients and human amyloid precursor protein (hAPP) transgenic mice. Amyloid-β oligomers reduced BRCA1 levels in primary neuronal cultures. In wild-type mice, knocking down neuronal BRCA1 in the dentate gyrus caused increased DNA double-strand breaks, neuronal shrinkage, synaptic plasticity impairments, and learning and memory deficits, but not apoptosis. Low levels of hAPP/Amyloid-β overexpression exacerbated these effects. Physiological neuronal activation increased BRCA1 levels, whereas stimulating predominantly extrasynaptic <jats:italic>N</jats:italic>-methyl-<jats:sc>D</jats:sc>-aspartate receptors promoted the proteasomal degradation of BRCA1. We conclude that BRCA1 is regulated by neuronal activity, protects the neuronal genome, and critically supports neuronal integrity and cognitive functions. Pathological accumulation of Aβ depletes neuronal BRCA1, which may contribute to cognitive deficits in AD.</jats:p> |
Leghmar, Kaoutar; Cenac, Nicolas; Rolland, Maude; Martin, Hélène; Rauwel, Benjamin; Bertrand-Michel, Justine; Faouder, Pauline Le; Bénard, Mélinda; Casper, Charlotte; Davrinche, Christian; Fournier, Thierry; Chavanas, Stéphane Cytomegalovirus Infection Triggers the Secretion of the PPARγ Agonists 15-Hydroxyeicosatetraenoic Acid (15-HETE) and 13-Hydroxyoctadecadienoic Acid (13-HODE) in Human Cytotrophoblasts and Placental Cultures Article de journal Dans: PLoS ONE, vol. 10, no. 7, 2015, ISSN: 1932-6203. @article{Leghmar2015,
title = {Cytomegalovirus Infection Triggers the Secretion of the PPARγ Agonists 15-Hydroxyeicosatetraenoic Acid (15-HETE) and 13-Hydroxyoctadecadienoic Acid (13-HODE) in Human Cytotrophoblasts and Placental Cultures},
author = {Kaoutar Leghmar and Nicolas Cenac and Maude Rolland and Hélène Martin and Benjamin Rauwel and Justine Bertrand-Michel and Pauline Le Faouder and Mélinda Bénard and Charlotte Casper and Christian Davrinche and Thierry Fournier and Stéphane Chavanas},
editor = {Juliet Spencer},
doi = {10.1371/journal.pone.0132627},
issn = {1932-6203},
year = {2015},
date = {2015-07-14},
urldate = {2015-07-14},
journal = {PLoS ONE},
volume = {10},
number = {7},
publisher = {Public Library of Science (PLoS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Li, Xiao-Jun; Liu, Xi-Juan; Yang, Bo; Fu, Ya-Ru; Zhao, Fei; Shen, Zhang-Zhou; Miao, Ling-Feng; Rayner, Simon; Chavanas, Stéphane; Zhu, Hua; Britt, William J.; Tang, Qiyi; McVoy, Michael A.; Luo, Min-Hua Human Cytomegalovirus Infection Dysregulates the Localization and Stability of NICD1 and Jag1 in Neural Progenitor Cells Article de journal Dans: J Virol, vol. 89, no. 13, p. 6792–6804, 2015, ISSN: 1098-5514. @article{Li2015,
title = {Human Cytomegalovirus Infection Dysregulates the Localization and Stability of NICD1 and Jag1 in Neural Progenitor Cells},
author = {Xiao-Jun Li and Xi-Juan Liu and Bo Yang and Ya-Ru Fu and Fei Zhao and Zhang-Zhou Shen and Ling-Feng Miao and Simon Rayner and Stéphane Chavanas and Hua Zhu and William J. Britt and Qiyi Tang and Michael A. McVoy and Min-Hua Luo},
editor = {R. M. Sandri-Goldin},
doi = {10.1128/jvi.00351-15},
issn = {1098-5514},
year = {2015},
date = {2015-07-00},
urldate = {2015-07-00},
journal = {J Virol},
volume = {89},
number = {13},
pages = {6792--6804},
publisher = {American Society for Microbiology},
abstract = {<jats:title>ABSTRACT</jats:title><jats:p>Human cytomegalovirus (HCMV) infection of the developing fetus frequently results in major neural developmental damage. In previous studies, HCMV was shown to downregulate neural progenitor/stem cell (NPC) markers and induce abnormal differentiation. As Notch signaling plays a vital role in the maintenance of stem cell status and is a switch that governs NPC differentiation, the effect of HCMV infection on the Notch signaling pathway in NPCs was investigated. HCMV downregulated mRNA levels of Notch1 and its ligand, Jag1, and reduced protein levels and altered the intracellular localization of Jag1 and the intracellular effector form of Notch1, NICD1. These effects required HCMV gene expression and appeared to be mediated through enhanced proteasomal degradation. Transient expression of the viral tegument proteins of pp71 and UL26 reduced NICD1 and Jag1 protein levels endogenously and exogenously. Given the critical role of Notch signaling in NPC growth and differentiation, these findings reveal important mechanisms by which HCMV disturbs neural cell development<jats:italic>in vitro</jats:italic>. Similar events<jats:italic>in vivo</jats:italic>may be associated with HCMV-mediated neuropathogenesis during congenital infection in the fetal brain.</jats:p><jats:p><jats:bold>IMPORTANCE</jats:bold>Congenital human cytomegalovirus (HCMV) infection is the leading cause of birth defects that primarily manifest as neurological disabilities. Neural progenitor cells (NPCs), key players in fetal brain development, are the most susceptible cell type for HCMV infection in the fetal brain. Studies have shown that NPCs are fully permissive for HCMV infection, which causes neural cell loss and premature differentiation, thereby perturbing NPC fate. Elucidation of virus-host interactions that govern NPC proliferation and differentiation is critical to understanding neuropathogenesis. The Notch signaling pathway is critical for maintaining stem cell status and functions as a switch for differentiation of NPCs. Our investigation into the impact of HCMV infection on this pathway revealed that HCMV dysregulates Notch signaling by altering expression of the Notch ligand Jag1, Notch1, and its active effector in NPCs. These results suggest a mechanism for the neuropathogenesis induced by HCMV infection that includes altered NPC differentiation and proliferation.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>ABSTRACT</jats:title><jats:p>Human cytomegalovirus (HCMV) infection of the developing fetus frequently results in major neural developmental damage. In previous studies, HCMV was shown to downregulate neural progenitor/stem cell (NPC) markers and induce abnormal differentiation. As Notch signaling plays a vital role in the maintenance of stem cell status and is a switch that governs NPC differentiation, the effect of HCMV infection on the Notch signaling pathway in NPCs was investigated. HCMV downregulated mRNA levels of Notch1 and its ligand, Jag1, and reduced protein levels and altered the intracellular localization of Jag1 and the intracellular effector form of Notch1, NICD1. These effects required HCMV gene expression and appeared to be mediated through enhanced proteasomal degradation. Transient expression of the viral tegument proteins of pp71 and UL26 reduced NICD1 and Jag1 protein levels endogenously and exogenously. Given the critical role of Notch signaling in NPC growth and differentiation, these findings reveal important mechanisms by which HCMV disturbs neural cell development<jats:italic>in vitro</jats:italic>. Similar events<jats:italic>in vivo</jats:italic>may be associated with HCMV-mediated neuropathogenesis during congenital infection in the fetal brain.</jats:p><jats:p><jats:bold>IMPORTANCE</jats:bold>Congenital human cytomegalovirus (HCMV) infection is the leading cause of birth defects that primarily manifest as neurological disabilities. Neural progenitor cells (NPCs), key players in fetal brain development, are the most susceptible cell type for HCMV infection in the fetal brain. Studies have shown that NPCs are fully permissive for HCMV infection, which causes neural cell loss and premature differentiation, thereby perturbing NPC fate. Elucidation of virus-host interactions that govern NPC proliferation and differentiation is critical to understanding neuropathogenesis. The Notch signaling pathway is critical for maintaining stem cell status and functions as a switch for differentiation of NPCs. Our investigation into the impact of HCMV infection on this pathway revealed that HCMV dysregulates Notch signaling by altering expression of the Notch ligand Jag1, Notch1, and its active effector in NPCs. These results suggest a mechanism for the neuropathogenesis induced by HCMV infection that includes altered NPC differentiation and proliferation.</jats:p> |
Bonnaud, Emilie M.; Szelechowski, Marion; Bétourné, Alexandre; Foret, Charlotte; Thouard, Anne; Gonzalez-Dunia, Daniel; Malnou, Cécile E. Borna Disease Virus Phosphoprotein Modulates Epigenetic Signaling in Neurons To Control Viral Replication Article de journal Dans: J Virol, vol. 89, no. 11, p. 5996–6008, 2015, ISSN: 1098-5514. @article{Bonnaud2015,
title = {Borna Disease Virus Phosphoprotein Modulates Epigenetic Signaling in Neurons To Control Viral Replication},
author = {Emilie M. Bonnaud and Marion Szelechowski and Alexandre Bétourné and Charlotte Foret and Anne Thouard and Daniel Gonzalez-Dunia and Cécile E. Malnou},
editor = {S. R. Ross},
doi = {10.1128/jvi.00454-15},
issn = {1098-5514},
year = {2015},
date = {2015-06-00},
urldate = {2015-06-00},
journal = {J Virol},
volume = {89},
number = {11},
pages = {5996--6008},
publisher = {American Society for Microbiology},
abstract = {<jats:title>ABSTRACT</jats:title>
<jats:p>Understanding the modalities of interaction of neurotropic viruses with their target cells represents a major challenge that may improve our knowledge of many human neurological disorders for which viral origin is suspected. Borna disease virus (BDV) represents an ideal model to analyze the molecular mechanisms of viral persistence in neurons and its consequences for neuronal homeostasis. It is now established that BDV ensures its long-term maintenance in infected cells through a stable interaction of viral components with the host cell chromatin, in particular, with core histones. This has led to our hypothesis that such an interaction may trigger epigenetic changes in the host cell. Here, we focused on histone acetylation, which plays key roles in epigenetic regulation of gene expression, notably for neurons. We performed a comparative analysis of histone acetylation patterns of neurons infected or not infected by BDV, which revealed that infection decreases histone acetylation on selected lysine residues. We showed that the BDV phosphoprotein (P) is responsible for these perturbations, even when it is expressed alone independently of the viral context, and that this action depends on its phosphorylation by protein kinase C. We also demonstrated that BDV P inhibits cellular histone acetyltransferase activities. Finally, by pharmacologically manipulating cellular acetylation levels, we observed that inhibiting cellular acetyl transferases reduces viral replication in cell culture. Our findings reveal that manipulation of cellular epigenetics by BDV could be a means to modulate viral replication and thus illustrate a fascinating example of virus-host cell interaction.</jats:p>
<jats:p>
<jats:bold>IMPORTANCE</jats:bold>
Persistent DNA viruses often subvert the mechanisms that regulate cellular chromatin dynamics, thereby benefitting from the resulting epigenetic changes to create a favorable milieu for their latent and persistent states. Here, we reasoned that Borna disease virus (BDV), the only RNA virus known to durably persist in the nucleus of infected cells, notably neurons, might employ a similar mechanism. In this study, we uncovered a novel modality of virus-cell interaction in which BDV phosphoprotein inhibits cellular histone acetylation by interfering with histone acetyltransferase activities. Manipulation of cellular histone acetylation is accompanied by a modulation of viral replication, revealing a perfect adaptation of this “ancient” virus to its host that may favor neuronal persistence and limit cellular damage.
</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>ABSTRACT</jats:title>
<jats:p>Understanding the modalities of interaction of neurotropic viruses with their target cells represents a major challenge that may improve our knowledge of many human neurological disorders for which viral origin is suspected. Borna disease virus (BDV) represents an ideal model to analyze the molecular mechanisms of viral persistence in neurons and its consequences for neuronal homeostasis. It is now established that BDV ensures its long-term maintenance in infected cells through a stable interaction of viral components with the host cell chromatin, in particular, with core histones. This has led to our hypothesis that such an interaction may trigger epigenetic changes in the host cell. Here, we focused on histone acetylation, which plays key roles in epigenetic regulation of gene expression, notably for neurons. We performed a comparative analysis of histone acetylation patterns of neurons infected or not infected by BDV, which revealed that infection decreases histone acetylation on selected lysine residues. We showed that the BDV phosphoprotein (P) is responsible for these perturbations, even when it is expressed alone independently of the viral context, and that this action depends on its phosphorylation by protein kinase C. We also demonstrated that BDV P inhibits cellular histone acetyltransferase activities. Finally, by pharmacologically manipulating cellular acetylation levels, we observed that inhibiting cellular acetyl transferases reduces viral replication in cell culture. Our findings reveal that manipulation of cellular epigenetics by BDV could be a means to modulate viral replication and thus illustrate a fascinating example of virus-host cell interaction.</jats:p>
<jats:p>
<jats:bold>IMPORTANCE</jats:bold>
Persistent DNA viruses often subvert the mechanisms that regulate cellular chromatin dynamics, thereby benefitting from the resulting epigenetic changes to create a favorable milieu for their latent and persistent states. Here, we reasoned that Borna disease virus (BDV), the only RNA virus known to durably persist in the nucleus of infected cells, notably neurons, might employ a similar mechanism. In this study, we uncovered a novel modality of virus-cell interaction in which BDV phosphoprotein inhibits cellular histone acetylation by interfering with histone acetyltransferase activities. Manipulation of cellular histone acetylation is accompanied by a modulation of viral replication, revealing a perfect adaptation of this “ancient” virus to its host that may favor neuronal persistence and limit cellular damage.
</jats:p> |
Fu, Ya-Ru; Liu, Xi-Juan; Li, Xiao-Jun; Shen, Zhang-zhou; Yang, Bo; Wu, Cong-Cong; Li, Jia-Fu; Miao, Ling-Feng; Ye, Han-Qing; Qiao, Guan-Hua; Rayner, Simon; Chavanas, Stéphane; Davrinche, Christian; Britt, William J.; Tang, Qiyi; McVoy, Michael; Mocarski, Edward; Luo, Min-Hua MicroRNA miR-21 Attenuates Human Cytomegalovirus Replication in Neural Cells by Targeting Cdc25a Article de journal Dans: J Virol, vol. 89, no. 2, p. 1070–1082, 2015, ISSN: 1098-5514. @article{Fu2015,
title = {MicroRNA miR-21 Attenuates Human Cytomegalovirus Replication in Neural Cells by Targeting Cdc25a},
author = {Ya-Ru Fu and Xi-Juan Liu and Xiao-Jun Li and Zhang-zhou Shen and Bo Yang and Cong-Cong Wu and Jia-Fu Li and Ling-Feng Miao and Han-Qing Ye and Guan-Hua Qiao and Simon Rayner and Stéphane Chavanas and Christian Davrinche and William J. Britt and Qiyi Tang and Michael McVoy and Edward Mocarski and Min-Hua Luo},
editor = {R. M. Sandri-Goldin},
doi = {10.1128/jvi.01740-14},
issn = {1098-5514},
year = {2015},
date = {2015-01-15},
urldate = {2015-01-15},
journal = {J Virol},
volume = {89},
number = {2},
pages = {1070--1082},
publisher = {American Society for Microbiology},
abstract = {<jats:title>ABSTRACT</jats:title><jats:p>Congenital human cytomegalovirus (HCMV) infection is a leading cause of birth defects, primarily manifesting as neurological disorders. HCMV infection alters expression of cellular microRNAs (miRs) and induces cell cycle arrest, which in turn modifies the cellular environment to favor virus replication. Previous observations found that HCMV infection reduces miR-21 expression in neural progenitor/stem cells (NPCs). Here, we show that infection of NPCs and U-251MG cells represses miR-21 while increasing the levels of Cdc25a, a cell cycle regulator and known target of miR-21. These opposing responses to infection prompted an investigation of the relationship between miR-21, Cdc25a, and viral replication. Overexpression of miR-21 in NPCs and U-251MG cells inhibited viral gene expression, genome replication, and production of infectious progeny, while shRNA-knockdown of miR-21 in U-251MG cells increased viral gene expression. In contrast, overexpression of Cdc25a in U-251MG cells increased viral gene expression and production of infectious progeny and overcame the inhibitory effects of miR-21 overexpression. Three viral gene products—IE1, pp71, and UL26—were shown to inhibit miR-21 expression at the transcriptional level. These results suggest that Cdc25a promotes HCMV replication and elevation of Cdc25a levels after HCMV infection are due in part to HCMV-mediated repression of miR-21. Thus, miR-21 is an intrinsic antiviral factor that is modulated by HCMV infection. This suggests a role for miR-21 downregulation in the neuropathogenesis of HCMV infection of the developing CNS.</jats:p><jats:p><jats:bold>IMPORTANCE</jats:bold>Human cytomegalovirus (HCMV) is a ubiquitous pathogen and has very high prevalence among population, especially in China, and congenital HCMV infection is a major cause for birth defects. Elucidating virus-host interactions that govern HCMV replication in neuronal cells is critical to understanding the neuropathogenesis of birth defects resulting from congenital infection. In this study, we confirm that HCMV infection downregulates miR-21 but upregulates Cdc25a. Further determined the negative effects of cellular miRNA miR-21 on HCMV replication in neural progenitor/stem cells and U-251MG glioblastoma/astrocytoma cells. More importantly, our results provide the first evidence that miR-21 negatively regulates HCMV replication by targeting Cdc25a, a vital cell cycle regulator. We further found that viral gene products of IE1, pp71, and UL26 play roles in inhibiting miR-21 expression, which in turn causes increases in Cdc25a and benefits HCMV replication. Thus, miR-21 appears to be an intrinsic antiviral factor that represents a potential target for therapeutic intervention.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>ABSTRACT</jats:title><jats:p>Congenital human cytomegalovirus (HCMV) infection is a leading cause of birth defects, primarily manifesting as neurological disorders. HCMV infection alters expression of cellular microRNAs (miRs) and induces cell cycle arrest, which in turn modifies the cellular environment to favor virus replication. Previous observations found that HCMV infection reduces miR-21 expression in neural progenitor/stem cells (NPCs). Here, we show that infection of NPCs and U-251MG cells represses miR-21 while increasing the levels of Cdc25a, a cell cycle regulator and known target of miR-21. These opposing responses to infection prompted an investigation of the relationship between miR-21, Cdc25a, and viral replication. Overexpression of miR-21 in NPCs and U-251MG cells inhibited viral gene expression, genome replication, and production of infectious progeny, while shRNA-knockdown of miR-21 in U-251MG cells increased viral gene expression. In contrast, overexpression of Cdc25a in U-251MG cells increased viral gene expression and production of infectious progeny and overcame the inhibitory effects of miR-21 overexpression. Three viral gene products—IE1, pp71, and UL26—were shown to inhibit miR-21 expression at the transcriptional level. These results suggest that Cdc25a promotes HCMV replication and elevation of Cdc25a levels after HCMV infection are due in part to HCMV-mediated repression of miR-21. Thus, miR-21 is an intrinsic antiviral factor that is modulated by HCMV infection. This suggests a role for miR-21 downregulation in the neuropathogenesis of HCMV infection of the developing CNS.</jats:p><jats:p><jats:bold>IMPORTANCE</jats:bold>Human cytomegalovirus (HCMV) is a ubiquitous pathogen and has very high prevalence among population, especially in China, and congenital HCMV infection is a major cause for birth defects. Elucidating virus-host interactions that govern HCMV replication in neuronal cells is critical to understanding the neuropathogenesis of birth defects resulting from congenital infection. In this study, we confirm that HCMV infection downregulates miR-21 but upregulates Cdc25a. Further determined the negative effects of cellular miRNA miR-21 on HCMV replication in neural progenitor/stem cells and U-251MG glioblastoma/astrocytoma cells. More importantly, our results provide the first evidence that miR-21 negatively regulates HCMV replication by targeting Cdc25a, a vital cell cycle regulator. We further found that viral gene products of IE1, pp71, and UL26 play roles in inhibiting miR-21 expression, which in turn causes increases in Cdc25a and benefits HCMV replication. Thus, miR-21 appears to be an intrinsic antiviral factor that represents a potential target for therapeutic intervention.</jats:p> |
2014
|
Szelechowski, Marion; Bétourné, Alexandre; Monnet, Yann; Ferré, Cécile A.; Thouard, Anne; Foret, Charlotte; Peyrin, Jean-Michel; Hunot, Stéphane; Gonzalez-Dunia, Daniel A viral peptide that targets mitochondria protects against neuronal degeneration in models of Parkinson’s disease Article de journal Dans: Nat Commun, vol. 5, no. 1, 2014, ISSN: 2041-1723. @article{Szelechowski2014,
title = {A viral peptide that targets mitochondria protects against neuronal degeneration in models of Parkinson’s disease},
author = {Marion Szelechowski and Alexandre Bétourné and Yann Monnet and Cécile A. Ferré and Anne Thouard and Charlotte Foret and Jean-Michel Peyrin and Stéphane Hunot and Daniel Gonzalez-Dunia},
doi = {10.1038/ncomms6181},
issn = {2041-1723},
year = {2014},
date = {2014-12-23},
urldate = {2014-12-23},
journal = {Nat Commun},
volume = {5},
number = {1},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2013
|
Charlier, Caroline M.; Wu, Yuan-Ju; Allart, Sophie; Malnou, Cécile E.; Schwemmle, Martin; Gonzalez-Dunia, Daniel Analysis of Borna Disease Virus Trafficking in Live Infected Cells by Using a Virus Encoding a Tetracysteine-Tagged P Protein Article de journal Dans: J Virol, vol. 87, no. 22, p. 12339–12348, 2013, ISSN: 1098-5514. @article{Charlier2013,
title = {Analysis of Borna Disease Virus Trafficking in Live Infected Cells by Using a Virus Encoding a Tetracysteine-Tagged P Protein},
author = {Caroline M. Charlier and Yuan-Ju Wu and Sophie Allart and Cécile E. Malnou and Martin Schwemmle and Daniel Gonzalez-Dunia},
doi = {10.1128/jvi.01127-13},
issn = {1098-5514},
year = {2013},
date = {2013-11-15},
urldate = {2013-11-15},
journal = {J Virol},
volume = {87},
number = {22},
pages = {12339--12348},
publisher = {American Society for Microbiology},
abstract = {<jats:title>ABSTRACT</jats:title>
<jats:p>Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus characterized by noncytolytic persistent infection and replication in the nuclei of infected cells. To gain further insight on the intracellular trafficking of BDV components during infection, we sought to generate recombinant BDV (rBDV) encoding fluorescent fusion viral proteins. We successfully rescued a virus bearing a tetracysteine tag fused to BDV-P protein, which allowed assessment of the intracellular distribution and dynamics of BDV using real-time live imaging. In persistently infected cells, viral nuclear inclusions, representing viral factories tethered to chromatin, appeared to be extremely static and stable, contrasting with a very rapid and active trafficking of BDV components in the cytoplasm. Photobleaching (fluorescence recovery after photobleaching [FRAP] and fluorescence loss in photobleaching [FLIP]) imaging approaches revealed that BDV components were permanently and actively exchanged between cellular compartments, including within viral inclusions, albeit with a fraction of BDV-P protein not mobile in these structures, presumably due to its association with viral and/or cellular proteins. We also obtained evidence for transfer of viral material between persistently infected cells, with routing of the transferred components toward the cell nucleus. Finally, coculture experiments with noninfected cells allowed visualization of cell-to-cell BDV transmission and movement of the incoming viral material toward the nucleus. Our data demonstrate the potential of tetracysteine-tagged recombinant BDV for virus tracking during infection, which may provide novel information on the BDV life cycle and on the modalities of its interaction with the nuclear environment during viral persistence.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>ABSTRACT</jats:title>
<jats:p>Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus characterized by noncytolytic persistent infection and replication in the nuclei of infected cells. To gain further insight on the intracellular trafficking of BDV components during infection, we sought to generate recombinant BDV (rBDV) encoding fluorescent fusion viral proteins. We successfully rescued a virus bearing a tetracysteine tag fused to BDV-P protein, which allowed assessment of the intracellular distribution and dynamics of BDV using real-time live imaging. In persistently infected cells, viral nuclear inclusions, representing viral factories tethered to chromatin, appeared to be extremely static and stable, contrasting with a very rapid and active trafficking of BDV components in the cytoplasm. Photobleaching (fluorescence recovery after photobleaching [FRAP] and fluorescence loss in photobleaching [FLIP]) imaging approaches revealed that BDV components were permanently and actively exchanged between cellular compartments, including within viral inclusions, albeit with a fraction of BDV-P protein not mobile in these structures, presumably due to its association with viral and/or cellular proteins. We also obtained evidence for transfer of viral material between persistently infected cells, with routing of the transferred components toward the cell nucleus. Finally, coculture experiments with noninfected cells allowed visualization of cell-to-cell BDV transmission and movement of the incoming viral material toward the nucleus. Our data demonstrate the potential of tetracysteine-tagged recombinant BDV for virus tracking during infection, which may provide novel information on the BDV life cycle and on the modalities of its interaction with the nuclear environment during viral persistence.</jats:p> |
Liblau, Roland S.; Gonzalez-Dunia, Daniel; Wiendl, Heinz; Zipp, Frauke Neurons as targets for T cells in the nervous system Article de journal Dans: Trends in Neurosciences, vol. 36, no. 6, p. 315–324, 2013, ISSN: 0166-2236. @article{Liblau2013,
title = {Neurons as targets for T cells in the nervous system},
author = {Roland S. Liblau and Daniel Gonzalez-Dunia and Heinz Wiendl and Frauke Zipp},
doi = {10.1016/j.tins.2013.01.008},
issn = {0166-2236},
year = {2013},
date = {2013-06-00},
urldate = {2013-06-00},
journal = {Trends in Neurosciences},
volume = {36},
number = {6},
pages = {315--324},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Suberbielle, Elsa; Sanchez, Pascal E; Kravitz, Alexxai V; Wang, Xin; Ho, Kaitlyn; Eilertson, Kirsten; Devidze, Nino; Kreitzer, Anatol C; Mucke, Lennart Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β Article de journal Dans: Nat Neurosci, vol. 16, no. 5, p. 613–621, 2013, ISSN: 1546-1726. @article{Suberbielle2013,
title = {Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β},
author = {Elsa Suberbielle and Pascal E Sanchez and Alexxai V Kravitz and Xin Wang and Kaitlyn Ho and Kirsten Eilertson and Nino Devidze and Anatol C Kreitzer and Lennart Mucke},
doi = {10.1038/nn.3356},
issn = {1546-1726},
year = {2013},
date = {2013-05-00},
urldate = {2013-05-00},
journal = {Nat Neurosci},
volume = {16},
number = {5},
pages = {613--621},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Szelechowski, Marion; Bergeron, Corinne; Gonzalez-Dunia, Daniel; Klonjkowski, Bernard Production and Purification of Non Replicative Canine Adenovirus Type 2 Derived Vectors Article de journal Dans: JoVE, no. 82, 2013, ISSN: 1940-087X. @article{Szelechowski2013,
title = {Production and Purification of Non Replicative Canine Adenovirus Type 2 Derived Vectors},
author = {Marion Szelechowski and Corinne Bergeron and Daniel Gonzalez-Dunia and Bernard Klonjkowski},
doi = {10.3791/50833},
issn = {1940-087X},
year = {2013},
date = {2013-00-00},
urldate = {2013-00-00},
journal = {JoVE},
number = {82},
publisher = {MyJove Corporation},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2011
|
Chevalier, Grégoire; Suberbielle, Elsa; Monnet, Céline; Duplan, Valérie; Martin-Blondel, Guillaume; Farrugia, Fanny; Masson, Gwendal Le; Liblau, Roland; Gonzalez-Dunia, Daniel Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection Article de journal Dans: PLoS Pathog, vol. 7, no. 11, 2011, ISSN: 1553-7374. @article{Chevalier2011,
title = {Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection},
author = {Grégoire Chevalier and Elsa Suberbielle and Céline Monnet and Valérie Duplan and Guillaume Martin-Blondel and Fanny Farrugia and Gwendal Le Masson and Roland Liblau and Daniel Gonzalez-Dunia},
editor = {Glenn F. Rall},
doi = {10.1371/journal.ppat.1002393},
issn = {1553-7374},
year = {2011},
date = {2011-11-17},
urldate = {2011-11-17},
journal = {PLoS Pathog},
volume = {7},
number = {11},
publisher = {Public Library of Science (PLoS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2010
|
Schmid, Sonja; Metz, Philippe; Prat, Christine M. A.; Gonzalez-Dunia, Daniel; Schwemmle, Martin Protein kinase C-dependent phosphorylation of Borna disease virus P protein is required for efficient viral spread Article de journal Dans: Arch Virol, vol. 155, no. 5, p. 789–793, 2010, ISSN: 1432-8798. @article{Schmid2010,
title = {Protein kinase C-dependent phosphorylation of Borna disease virus P protein is required for efficient viral spread},
author = {Sonja Schmid and Philippe Metz and Christine M. A. Prat and Daniel Gonzalez-Dunia and Martin Schwemmle},
doi = {10.1007/s00705-010-0645-9},
issn = {1432-8798},
year = {2010},
date = {2010-05-00},
urldate = {2010-05-00},
journal = {Arch Virol},
volume = {155},
number = {5},
pages = {789--793},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2009
|
Prat, Christine M. A.; Schmid, Sonja; Farrugia, Fanny; Cenac, Nicolas; Masson, Gwendal Le; Schwemmle, Martin; Gonzalez-Dunia, Daniel Mutation of the Protein Kinase C Site in Borna Disease Virus Phosphoprotein Abrogates Viral Interference with Neuronal Signaling and Restores Normal Synaptic Activity Article de journal Dans: PLoS Pathog, vol. 5, no. 5, 2009, ISSN: 1553-7374. @article{Prat2009,
title = {Mutation of the Protein Kinase C Site in Borna Disease Virus Phosphoprotein Abrogates Viral Interference with Neuronal Signaling and Restores Normal Synaptic Activity},
author = {Christine M. A. Prat and Sonja Schmid and Fanny Farrugia and Nicolas Cenac and Gwendal Le Masson and Martin Schwemmle and Daniel Gonzalez-Dunia},
editor = {Raul Andino},
doi = {10.1371/journal.ppat.1000425},
issn = {1553-7374},
year = {2009},
date = {2009-05-08},
urldate = {2009-05-08},
journal = {PLoS Pathog},
volume = {5},
number = {5},
publisher = {Public Library of Science (PLoS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|