2024
|
Morandi, Elena; Adoue, Véronique; Bernard, Isabelle; Friebel, Ekaterina; Nunez, Nicolas; Aubert, Yann; Masson, Frederick; Dejean, Anne S; Becher, Burkhard; Astier, Anne; Martinet, Ludovic; Saoudi, Abdelhadi Impact of the Multiple Sclerosis-Associated Genetic Variant CD226 Gly307Ser on Human CD8 T-Cell Functions Article de journal Dans: Neurol Neuroimmunol Neuroinflammation, vol. 11, no. 6, 2024. @article{nokey,
title = {Impact of the Multiple Sclerosis-Associated Genetic Variant CD226 Gly307Ser on Human CD8 T-Cell Functions},
author = {Elena Morandi and Véronique Adoue and Isabelle Bernard and Ekaterina Friebel and Nicolas Nunez and Yann Aubert and Frederick Masson and Anne S Dejean and Burkhard Becher and Anne Astier and Ludovic Martinet and Abdelhadi Saoudi},
doi = {10.1212/NXI.0000000000200306},
year = {2024},
date = {2024-09-04},
urldate = {2024-09-04},
journal = {Neurol Neuroimmunol Neuroinflammation},
volume = {11},
number = {6},
abstract = {Background and Objectives
The rs763361 nonsynonymous variant in the CD226 gene, which results in a glycine-to-serine substitution at position 307 of the CD226 protein, has been implicated as a risk factor of various immune-mediated diseases, including multiple sclerosis (MS). Compelling evidence suggests that this allele may play a significant role in predisposing individuals to MS by decreasing the immune-regulatory capacity of Treg cells and increasing the proinflammatory potential of effector CD4 T cells. However, the impact of this CD226 gene variant on CD8 T-cell functions, a population that also plays a key role in MS, remains to be determined.
Methods
To study whether the CD226 risk variant affects human CD8 T-cell functions, we used CD8 T cells isolated from peripheral blood mononuclear cell of 16 age-matched healthy donors homozygous for either the protective or the risk allele of CD226. We characterized these CD8 T cells on T-cell receptor (TCR) stimulation using high-parametric flow cytometry and bulk RNAseq and through characterization of canonical signaling pathways and cytokine production.
Results
On TCR engagement, the phenotype of ex vivo CD8 T cells bearing the protective (CD226-307Gly) or the risk (CD226-307Ser) allele of CD226 was largely overlapping. However, the transcriptomic signature of CD8 T cells from the donors carrying the risk allele presented an enrichment in TCR, JAK/STAT, and IFNγ signaling. We next found that the CD226-307Ser risk allele leads to a selective increase in the phosphorylation of the mitogen-activated protein kinases extracellular signal–regulated kinases 1 and 2 (ERK1/2) associated with enhanced phosphorylation of STAT4 and increased production of IFNγ.
Discussion
Our data suggest that the CD226-307Ser risk variant imposes immune dysregulation by increasing the pathways related to IFNγ signaling in CD8 T cells, thereby contributing to the risk of developing chronic inflammation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Background and Objectives
The rs763361 nonsynonymous variant in the CD226 gene, which results in a glycine-to-serine substitution at position 307 of the CD226 protein, has been implicated as a risk factor of various immune-mediated diseases, including multiple sclerosis (MS). Compelling evidence suggests that this allele may play a significant role in predisposing individuals to MS by decreasing the immune-regulatory capacity of Treg cells and increasing the proinflammatory potential of effector CD4 T cells. However, the impact of this CD226 gene variant on CD8 T-cell functions, a population that also plays a key role in MS, remains to be determined.
Methods
To study whether the CD226 risk variant affects human CD8 T-cell functions, we used CD8 T cells isolated from peripheral blood mononuclear cell of 16 age-matched healthy donors homozygous for either the protective or the risk allele of CD226. We characterized these CD8 T cells on T-cell receptor (TCR) stimulation using high-parametric flow cytometry and bulk RNAseq and through characterization of canonical signaling pathways and cytokine production.
Results
On TCR engagement, the phenotype of ex vivo CD8 T cells bearing the protective (CD226-307Gly) or the risk (CD226-307Ser) allele of CD226 was largely overlapping. However, the transcriptomic signature of CD8 T cells from the donors carrying the risk allele presented an enrichment in TCR, JAK/STAT, and IFNγ signaling. We next found that the CD226-307Ser risk allele leads to a selective increase in the phosphorylation of the mitogen-activated protein kinases extracellular signal–regulated kinases 1 and 2 (ERK1/2) associated with enhanced phosphorylation of STAT4 and increased production of IFNγ.
Discussion
Our data suggest that the CD226-307Ser risk variant imposes immune dysregulation by increasing the pathways related to IFNγ signaling in CD8 T cells, thereby contributing to the risk of developing chronic inflammation. |
Kari, Saniya; Bucciarelli, Florence; Angles, Thibault; Oster, Anne-Cecile; Cauboue, Pauline; Laviolette, Karl; Mougenot, Madeline; Morandi, Elena; Bernard, Isabelle; Pignolet, Beatrice; Bost, Chloé; Thomas, Joelle; Nogueira, Leonor; Saoudi, Abdelhadi; Liblau, Roland; Astier, Anne L Increased levels of circulating soluble CD226 in multiple sclerosis Article de journal Dans: Mult Scler, p. 13524585241234489, 2024, ISSN: 1477-0970. @article{pmid38424741,
title = {Increased levels of circulating soluble CD226 in multiple sclerosis},
author = {Saniya Kari and Florence Bucciarelli and Thibault Angles and Anne-Cecile Oster and Pauline Cauboue and Karl Laviolette and Madeline Mougenot and Elena Morandi and Isabelle Bernard and Beatrice Pignolet and Chloé Bost and Joelle Thomas and Leonor Nogueira and Abdelhadi Saoudi and Roland Liblau and Anne L Astier},
doi = {10.1177/13524585241234489},
issn = {1477-0970},
year = {2024},
date = {2024-02-01},
urldate = {2024-02-01},
journal = {Mult Scler},
pages = {13524585241234489},
abstract = {BACKGROUND: The glycoprotein CD226 plays a key role in regulating immune cell function. Soluble CD226 (sCD226) is increased in sera of patients with several chronic inflammatory diseases but its levels in neuroinflammatory diseases such as multiple sclerosis (MS) are unknown.nnOBJECTIVE: To investigate the presence and functional implications of sCD226 in persons with multiple sclerosis (pwMS) and other neurological diseases.nnMETHODS: The mechanisms of sCD226 production were first investigated by analyzing CD226 surface expression levels and supernatants of CD3/CD226-coactivated T cells. The role of sCD226 on dendritic cell maturation was evaluated. The concentration of sCD226 in the sera from healthy donors (HD), pwMS, neuromyelitis optica (NMO), and Alzheimer's disease (AD) was measured.nnRESULTS: CD3/CD226-costimulation induced CD226 shedding. Addition of sCD226 to dendritic cells during their maturation led to an increased production of the pro-inflammatory cytokine interleukin (IL)-23. We observed a significant increase in sCD226 in sera from pwMS and NMO compared to HD and AD. In MS, levels were increased in both relapsing-remitting multiple sclerosis (RRMS) and secondary-progressive multiple sclerosis (SPMS) compared to clinically isolated syndrome (CIS).nnCONCLUSION: Our data suggest that T-cell activation leads to release of sCD226 that could promote inflammation and raises the possibility of using sCD226 as a biomarker for neuroinflammation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: The glycoprotein CD226 plays a key role in regulating immune cell function. Soluble CD226 (sCD226) is increased in sera of patients with several chronic inflammatory diseases but its levels in neuroinflammatory diseases such as multiple sclerosis (MS) are unknown.nnOBJECTIVE: To investigate the presence and functional implications of sCD226 in persons with multiple sclerosis (pwMS) and other neurological diseases.nnMETHODS: The mechanisms of sCD226 production were first investigated by analyzing CD226 surface expression levels and supernatants of CD3/CD226-coactivated T cells. The role of sCD226 on dendritic cell maturation was evaluated. The concentration of sCD226 in the sera from healthy donors (HD), pwMS, neuromyelitis optica (NMO), and Alzheimer's disease (AD) was measured.nnRESULTS: CD3/CD226-costimulation induced CD226 shedding. Addition of sCD226 to dendritic cells during their maturation led to an increased production of the pro-inflammatory cytokine interleukin (IL)-23. We observed a significant increase in sCD226 in sera from pwMS and NMO compared to HD and AD. In MS, levels were increased in both relapsing-remitting multiple sclerosis (RRMS) and secondary-progressive multiple sclerosis (SPMS) compared to clinically isolated syndrome (CIS).nnCONCLUSION: Our data suggest that T-cell activation leads to release of sCD226 that could promote inflammation and raises the possibility of using sCD226 as a biomarker for neuroinflammation. |
Joulia, Emeline; Michieletto, Michaël F.; Agesta, Arantxa; Peillex, Cindy; Girault, Virginie; Dorze, Anne-Louise Le; Peroceschi, Romain; Bucciarelli, Florence; Szelechowski, Marion; Chaubet, Adeline; Hakim, Nawad; Marrocco, Rémi; Lhuillier, Emeline; Lebeurrier, Manuel; Argüello, Rafael J.; Saoudi, Abdelhadi; Costa, Hicham El; Adoue, Veronique; Walzer, Thierry; Sarry, Jean-Emmanuel; Dejean, Anne S. Eomes-dependent mitochondrial regulation promotes survival of pathogenic CD4+ T cells during inflammation Article de journal Dans: Journal of Experimental Medicine, vol. 221, no. 2, 2024, ISSN: 0022-1007. @article{nokey,
title = {Eomes-dependent mitochondrial regulation promotes survival of pathogenic CD4+ T cells during inflammation},
author = {Emeline Joulia and Michaël F. Michieletto and Arantxa Agesta and Cindy Peillex and Virginie Girault and Anne-Louise Le Dorze and Romain Peroceschi and Florence Bucciarelli and Marion Szelechowski and Adeline Chaubet and Nawad Hakim and Rémi Marrocco and Emeline Lhuillier and Manuel Lebeurrier and Rafael J. Argüello and Abdelhadi Saoudi and Hicham El Costa and Veronique Adoue and Thierry Walzer and Jean-Emmanuel Sarry and Anne S. Dejean},
doi = {10.1084/jem.20230449},
issn = {0022-1007},
year = {2024},
date = {2024-01-08},
journal = {Journal of Experimental Medicine},
volume = {221},
number = {2},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2023
|
Liblau, Roland S; Latorre, Daniela; Kornum, Birgitte R; Dauvilliers, Yves; Mignot, Emmanuel J The immunopathogenesis of narcolepsy type 1 Article de journal Dans: Nat Rev Immunol, 2023, ISSN: 1474-1741. @article{pmid37400646,
title = {The immunopathogenesis of narcolepsy type 1},
author = {Roland S Liblau and Daniela Latorre and Birgitte R Kornum and Yves Dauvilliers and Emmanuel J Mignot},
doi = {10.1038/s41577-023-00902-9},
issn = {1474-1741},
year = {2023},
date = {2023-07-01},
urldate = {2023-07-01},
journal = {Nat Rev Immunol},
abstract = {Narcolepsy type 1 (NT1) is a chronic sleep disorder resulting from the loss of a small population of hypothalamic neurons that produce wake-promoting hypocretin (HCRT; also known as orexin) peptides. An immune-mediated pathology for NT1 has long been suspected given its exceptionally tight association with the MHC class II allele HLA-DQB1*06:02, as well as recent genetic evidence showing associations with polymorphisms of T cell receptor genes and other immune-relevant loci and the increased incidence of NT1 that has been observed after vaccination with the influenza vaccine Pandemrix. The search for both self-antigens and foreign antigens recognized by the pathogenic T cell response in NT1 is ongoing. Increased T cell reactivity against HCRT has been consistently reported in patients with NT1, but data demonstrating a primary role for T cells in neuronal destruction are currently lacking. Animal models are providing clues regarding the roles of autoreactive CD4 and CD8 T cells in the disease. Elucidation of the pathogenesis of NT1 will allow for the development of targeted immunotherapies at disease onset and could serve as a model for other immune-mediated neurological diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Narcolepsy type 1 (NT1) is a chronic sleep disorder resulting from the loss of a small population of hypothalamic neurons that produce wake-promoting hypocretin (HCRT; also known as orexin) peptides. An immune-mediated pathology for NT1 has long been suspected given its exceptionally tight association with the MHC class II allele HLA-DQB1*06:02, as well as recent genetic evidence showing associations with polymorphisms of T cell receptor genes and other immune-relevant loci and the increased incidence of NT1 that has been observed after vaccination with the influenza vaccine Pandemrix. The search for both self-antigens and foreign antigens recognized by the pathogenic T cell response in NT1 is ongoing. Increased T cell reactivity against HCRT has been consistently reported in patients with NT1, but data demonstrating a primary role for T cells in neuronal destruction are currently lacking. Animal models are providing clues regarding the roles of autoreactive CD4 and CD8 T cells in the disease. Elucidation of the pathogenesis of NT1 will allow for the development of targeted immunotherapies at disease onset and could serve as a model for other immune-mediated neurological diseases. |
Geeraerts, Thomas; Guilbeau-Frugier, Céline; Garcia, Cédric; Memier, Vincent; Raposo, Nicolas; Bonneville, Fabrice; Gales, Céline; Darcourt, Jean; Voisin, Sophie; Ribes, Agnès; Piel-Julian, Marie; Bounes, Fanny; Albucher, Jean François; Roux, Franck-Emmanuel; Izopet, Jacques; Telmon, Norbert; Olivot, Jean Marc; Sié, Pierre; Bauer, Jan; Payrastre, Bernard; Liblau, Roland S Immunohistologic Features of Cerebral Venous Thrombosis Due to Vaccine-Induced Immune Thrombotic Thrombocytopenia Article de journal Dans: Neurol Neuroimmunol Neuroinflamm, vol. 10, no. 4, 2023, ISSN: 2332-7812. @article{pmid37236806,
title = {Immunohistologic Features of Cerebral Venous Thrombosis Due to Vaccine-Induced Immune Thrombotic Thrombocytopenia},
author = {Thomas Geeraerts and Céline Guilbeau-Frugier and Cédric Garcia and Vincent Memier and Nicolas Raposo and Fabrice Bonneville and Céline Gales and Jean Darcourt and Sophie Voisin and Agnès Ribes and Marie Piel-Julian and Fanny Bounes and Jean François Albucher and Franck-Emmanuel Roux and Jacques Izopet and Norbert Telmon and Jean Marc Olivot and Pierre Sié and Jan Bauer and Bernard Payrastre and Roland S Liblau},
doi = {10.1212/NXI.0000000000200127},
issn = {2332-7812},
year = {2023},
date = {2023-07-01},
urldate = {2023-07-01},
journal = {Neurol Neuroimmunol Neuroinflamm},
volume = {10},
number = {4},
abstract = {OBJECTIVES: Vaccine-induced immune thrombotic thrombocytopenia (VITT), a recently described entity characterized by thrombosis at unusual locations such as cerebral venous sinus and splanchnic vein, has been rarely described after adenoviral-encoded COVID-19 vaccines. In this study, we report the immunohistological correlates in 3 fatal cases of cerebral venous thrombosis related to VITT analyzed at an academic medical center.nnMETHODS: Detailed neuropathologic studies were performed in 3 cases of cerebral venous thrombosis related to VITT after adenoviral COVID-19 vaccination.nnRESULTS: Autopsy revealed extensive cerebral vein thrombosis in all 3 cases. Polarized thrombi were observed with a high density of neutrophils in the core and a low density in the tail. Endothelial cells adjacent to the thrombus were largely destroyed. Markers of neutrophil extracellular trap and complement activation were present at the border and within the cerebral vein thrombi. SARS-CoV-2 spike protein was detected within the thrombus and in the adjacent vessel wall.nnDISCUSSION: Data indicate that neutrophils and complement activation associated with antispike immunity triggered by the vaccine is probably involved in the disease process.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
OBJECTIVES: Vaccine-induced immune thrombotic thrombocytopenia (VITT), a recently described entity characterized by thrombosis at unusual locations such as cerebral venous sinus and splanchnic vein, has been rarely described after adenoviral-encoded COVID-19 vaccines. In this study, we report the immunohistological correlates in 3 fatal cases of cerebral venous thrombosis related to VITT analyzed at an academic medical center.nnMETHODS: Detailed neuropathologic studies were performed in 3 cases of cerebral venous thrombosis related to VITT after adenoviral COVID-19 vaccination.nnRESULTS: Autopsy revealed extensive cerebral vein thrombosis in all 3 cases. Polarized thrombi were observed with a high density of neutrophils in the core and a low density in the tail. Endothelial cells adjacent to the thrombus were largely destroyed. Markers of neutrophil extracellular trap and complement activation were present at the border and within the cerebral vein thrombi. SARS-CoV-2 spike protein was detected within the thrombus and in the adjacent vessel wall.nnDISCUSSION: Data indicate that neutrophils and complement activation associated with antispike immunity triggered by the vaccine is probably involved in the disease process. |
Gonzalez-Fierro, Carmen; Fonte, Coralie; Dufourd, Eloïse; Cazaentre, Vincent; Aydin, Sidar; Engelhardt, Britta; Caspi, Rachel R; Xu, Biying; Martin-Blondel, Guillaume; Spicer, Julie A; Trapani, Joseph A; Bauer, Jan; Liblau, Roland S; Bost, Chloé Effects of a Small-Molecule Perforin Inhibitor in a Mouse Model of CD8 T Cell-Mediated Neuroinflammation Article de journal Dans: Neurol Neuroimmunol Neuroinflamm, vol. 10, no. 4, 2023, ISSN: 2332-7812. @article{pmid37080596,
title = {Effects of a Small-Molecule Perforin Inhibitor in a Mouse Model of CD8 T Cell-Mediated Neuroinflammation},
author = {Carmen Gonzalez-Fierro and Coralie Fonte and Eloïse Dufourd and Vincent Cazaentre and Sidar Aydin and Britta Engelhardt and Rachel R Caspi and Biying Xu and Guillaume Martin-Blondel and Julie A Spicer and Joseph A Trapani and Jan Bauer and Roland S Liblau and Chloé Bost},
doi = {10.1212/NXI.0000000000200117},
issn = {2332-7812},
year = {2023},
date = {2023-07-01},
urldate = {2023-07-01},
journal = {Neurol Neuroimmunol Neuroinflamm},
volume = {10},
number = {4},
abstract = {BACKGROUND AND OBJECTIVES: Alteration of the blood-brain barrier (BBB) at the interface between blood and CNS parenchyma is prominent in most neuroinflammatory diseases. In several neurologic diseases, including cerebral malaria and Susac syndrome, a CD8 T cell-mediated targeting of endothelial cells of the BBB (BBB-ECs) has been implicated in pathogenesis.nnMETHODS: In this study, we used an experimental mouse model to evaluate the ability of a small-molecule perforin inhibitor to prevent neuroinflammation resulting from cytotoxic CD8 T cell-mediated damage of BBB-ECs.nnRESULTS: Using an in vitro coculture system, we first identified perforin as an essential molecule for killing of BBB-ECs by CD8 T cells. We then found that short-term pharmacologic inhibition of perforin commencing after disease onset restored motor function and inhibited the neuropathology. Perforin inhibition resulted in preserved BBB-EC viability, maintenance of the BBB, and reduced CD8 T-cell accumulation in the brain and retina.nnDISCUSSION: Therefore, perforin-dependent cytotoxicity plays a key role in the death of BBB-ECs inflicted by autoreactive CD8 T cells in a preclinical model and potentially represents a therapeutic target for CD8 T cell-mediated neuroinflammatory diseases, such as cerebral malaria and Susac syndrome.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND AND OBJECTIVES: Alteration of the blood-brain barrier (BBB) at the interface between blood and CNS parenchyma is prominent in most neuroinflammatory diseases. In several neurologic diseases, including cerebral malaria and Susac syndrome, a CD8 T cell-mediated targeting of endothelial cells of the BBB (BBB-ECs) has been implicated in pathogenesis.nnMETHODS: In this study, we used an experimental mouse model to evaluate the ability of a small-molecule perforin inhibitor to prevent neuroinflammation resulting from cytotoxic CD8 T cell-mediated damage of BBB-ECs.nnRESULTS: Using an in vitro coculture system, we first identified perforin as an essential molecule for killing of BBB-ECs by CD8 T cells. We then found that short-term pharmacologic inhibition of perforin commencing after disease onset restored motor function and inhibited the neuropathology. Perforin inhibition resulted in preserved BBB-EC viability, maintenance of the BBB, and reduced CD8 T-cell accumulation in the brain and retina.nnDISCUSSION: Therefore, perforin-dependent cytotoxicity plays a key role in the death of BBB-ECs inflicted by autoreactive CD8 T cells in a preclinical model and potentially represents a therapeutic target for CD8 T cell-mediated neuroinflammatory diseases, such as cerebral malaria and Susac syndrome. |
Seifinejad, Ali; Ramosaj, Mergim; Shan, Ling; Li, Sha; Possovre, Marie-Laure; Pfister, Corinne; Fronczek, Rolf; Garrett-Sinha, Lee A; Frieser, David; Honda, Makoto; Arribat, Yoan; Grepper, Dogan; Amati, Francesca; Picot, Marie; Agnoletto, Andrea; Iseli, Christian; Chartrel, Nicolas; Liblau, Roland; Lammers, Gert J; Vassalli, Anne; Tafti, Mehdi Epigenetic silencing of selected hypothalamic neuropeptides in narcolepsy with cataplexy Article de journal Dans: Proc Natl Acad Sci U S A, vol. 120, no. 19, p. e2220911120, 2023, ISSN: 1091-6490. @article{pmid37126681,
title = {Epigenetic silencing of selected hypothalamic neuropeptides in narcolepsy with cataplexy},
author = {Ali Seifinejad and Mergim Ramosaj and Ling Shan and Sha Li and Marie-Laure Possovre and Corinne Pfister and Rolf Fronczek and Lee A Garrett-Sinha and David Frieser and Makoto Honda and Yoan Arribat and Dogan Grepper and Francesca Amati and Marie Picot and Andrea Agnoletto and Christian Iseli and Nicolas Chartrel and Roland Liblau and Gert J Lammers and Anne Vassalli and Mehdi Tafti},
doi = {10.1073/pnas.2220911120},
issn = {1091-6490},
year = {2023},
date = {2023-05-01},
urldate = {2023-05-01},
journal = {Proc Natl Acad Sci U S A},
volume = {120},
number = {19},
pages = {e2220911120},
abstract = {Narcolepsy with cataplexy is a sleep disorder caused by deficiency in the hypothalamic neuropeptide hypocretin/orexin (HCRT), unanimously believed to result from autoimmune destruction of hypocretin-producing neurons. HCRT deficiency can also occur in secondary forms of narcolepsy and be only temporary, suggesting it can occur without irreversible neuronal loss. The recent discovery that narcolepsy patients also show loss of hypothalamic (corticotropin-releasing hormone) CRH-producing neurons suggests that other mechanisms than cell-specific autoimmune attack, are involved. Here, we identify the HCRT cell-colocalized neuropeptide QRFP as the best marker of HCRT neurons. We show that if HCRT neurons are ablated in mice, in addition to transcript is also lost in the lateral hypothalamus, while in mice where only the gene is inactivated is unchanged. Similarly, postmortem hypothalamic tissues of narcolepsy patients show preserved expression, suggesting the neurons are present but fail to actively produce HCRT. We show that the promoter of the gene of patients exhibits hypermethylation at a methylation-sensitive and evolutionary-conserved PAX5:ETS1 transcription factor-binding site, suggesting the gene is subject to transcriptional silencing. We show also that in addition to HCRT, and Dynorphin () gene promoters, exhibit hypermethylation in the hypothalamus of patients. Altogether, we propose that , , and are epigenetically silenced by a hypothalamic assault (inflammation) in narcolepsy patients, without concurrent cell death. Since methylation is reversible, our findings open the prospect of reversing or curing narcolepsy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Narcolepsy with cataplexy is a sleep disorder caused by deficiency in the hypothalamic neuropeptide hypocretin/orexin (HCRT), unanimously believed to result from autoimmune destruction of hypocretin-producing neurons. HCRT deficiency can also occur in secondary forms of narcolepsy and be only temporary, suggesting it can occur without irreversible neuronal loss. The recent discovery that narcolepsy patients also show loss of hypothalamic (corticotropin-releasing hormone) CRH-producing neurons suggests that other mechanisms than cell-specific autoimmune attack, are involved. Here, we identify the HCRT cell-colocalized neuropeptide QRFP as the best marker of HCRT neurons. We show that if HCRT neurons are ablated in mice, in addition to transcript is also lost in the lateral hypothalamus, while in mice where only the gene is inactivated is unchanged. Similarly, postmortem hypothalamic tissues of narcolepsy patients show preserved expression, suggesting the neurons are present but fail to actively produce HCRT. We show that the promoter of the gene of patients exhibits hypermethylation at a methylation-sensitive and evolutionary-conserved PAX5:ETS1 transcription factor-binding site, suggesting the gene is subject to transcriptional silencing. We show also that in addition to HCRT, and Dynorphin () gene promoters, exhibit hypermethylation in the hypothalamus of patients. Altogether, we propose that , , and are epigenetically silenced by a hypothalamic assault (inflammation) in narcolepsy patients, without concurrent cell death. Since methylation is reversible, our findings open the prospect of reversing or curing narcolepsy. |
Aydin, Sidar; Pareja, Javier; Schallenberg, Vivianne M; Klopstein, Armelle; Gruber, Thomas; Page, Nicolas; Bouillet, Elisa; Blanchard, Nicolas; Liblau, Roland; Körbelin, Jakob; Schwaninger, Markus; Johnson, Aaron J; Schenk, Mirjam; Deutsch, Urban; Merkler, Doron; Engelhardt, Britta Antigen recognition detains CD8 T cells at the blood-brain barrier and contributes to its breakdown Article de journal Dans: Nat Commun, vol. 14, no. 1, p. 3106, 2023, ISSN: 2041-1723. @article{pmid37253744,
title = {Antigen recognition detains CD8 T cells at the blood-brain barrier and contributes to its breakdown},
author = {Sidar Aydin and Javier Pareja and Vivianne M Schallenberg and Armelle Klopstein and Thomas Gruber and Nicolas Page and Elisa Bouillet and Nicolas Blanchard and Roland Liblau and Jakob Körbelin and Markus Schwaninger and Aaron J Johnson and Mirjam Schenk and Urban Deutsch and Doron Merkler and Britta Engelhardt},
doi = {10.1038/s41467-023-38703-2},
issn = {2041-1723},
year = {2023},
date = {2023-05-01},
urldate = {2023-05-01},
journal = {Nat Commun},
volume = {14},
number = {1},
pages = {3106},
abstract = {Blood-brain barrier (BBB) breakdown and immune cell infiltration into the central nervous system (CNS) are early hallmarks of multiple sclerosis (MS). High numbers of CD8 T cells are found in MS lesions, and antigen (Ag) presentation at the BBB has been proposed to promote CD8 T cell entry into the CNS. Here, we show that brain endothelial cells process and cross-present Ag, leading to effector CD8 T cell differentiation. Under physiological flow in vitro, endothelial Ag presentation prevented CD8 T cell crawling and diapedesis resulting in brain endothelial cell apoptosis and BBB breakdown. Brain endothelial Ag presentation in vivo was limited due to Ag uptake by CNS-resident macrophages but still reduced motility of Ag-specific CD8 T cells within CNS microvessels. MHC class I-restricted Ag presentation at the BBB during neuroinflammation thus prohibits CD8 T cell entry into the CNS and triggers CD8 T cell-mediated focal BBB breakdown.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Blood-brain barrier (BBB) breakdown and immune cell infiltration into the central nervous system (CNS) are early hallmarks of multiple sclerosis (MS). High numbers of CD8 T cells are found in MS lesions, and antigen (Ag) presentation at the BBB has been proposed to promote CD8 T cell entry into the CNS. Here, we show that brain endothelial cells process and cross-present Ag, leading to effector CD8 T cell differentiation. Under physiological flow in vitro, endothelial Ag presentation prevented CD8 T cell crawling and diapedesis resulting in brain endothelial cell apoptosis and BBB breakdown. Brain endothelial Ag presentation in vivo was limited due to Ag uptake by CNS-resident macrophages but still reduced motility of Ag-specific CD8 T cells within CNS microvessels. MHC class I-restricted Ag presentation at the BBB during neuroinflammation thus prohibits CD8 T cell entry into the CNS and triggers CD8 T cell-mediated focal BBB breakdown. |
Martin-Blondel, Guillaume; Marcelin, Anne-Geneviève; Soulié, Cathia; Kaisaridi, Sofia; Lusivika-Nzinga, Clovis; Zafilaza, Karen; Dorival, Céline; Nailler, Laura; Boston, Anaïs; Ronchetti, Anne-Marie; Melenotte, Cléa; Cabié, André; Choquet, Christophe; Trinh-Duc, Albert; Lacombe, Karine; Gaube, Géraldine; Coustillères, François; Pourcher, Valérie; Martellosio, Jean-Philippe; Peiffer-Smadja, Nathan; Chauveau, Marie; Housset, Pierre; Piroth, Lionel; Devaux, Mathilde; Pialoux, Gilles; Martin, Aurélie; Dubee, Vincent; Frey, Jérôme; Bot, Audrey Le; Cazanave, Charles; Petua, Philippe; Liblau, Roland; Carrat, Fabrice; Yordanov, Youri Time to negative PCR conversion amongst high-risk patients with mild-to-moderate Omicron BA.1 and BA.2 COVID-19 treated with sotrovimab or nirmatrelvir Article de journal Dans: Clin Microbiol Infect, vol. 29, no. 4, p. 543.e5–543.e9, 2023, ISSN: 1469-0691. @article{pmid36586513,
title = {Time to negative PCR conversion amongst high-risk patients with mild-to-moderate Omicron BA.1 and BA.2 COVID-19 treated with sotrovimab or nirmatrelvir},
author = {Guillaume Martin-Blondel and Anne-Geneviève Marcelin and Cathia Soulié and Sofia Kaisaridi and Clovis Lusivika-Nzinga and Karen Zafilaza and Céline Dorival and Laura Nailler and Anaïs Boston and Anne-Marie Ronchetti and Cléa Melenotte and André Cabié and Christophe Choquet and Albert Trinh-Duc and Karine Lacombe and Géraldine Gaube and François Coustillères and Valérie Pourcher and Jean-Philippe Martellosio and Nathan Peiffer-Smadja and Marie Chauveau and Pierre Housset and Lionel Piroth and Mathilde Devaux and Gilles Pialoux and Aurélie Martin and Vincent Dubee and Jérôme Frey and Audrey Le Bot and Charles Cazanave and Philippe Petua and Roland Liblau and Fabrice Carrat and Youri Yordanov},
doi = {10.1016/j.cmi.2022.12.016},
issn = {1469-0691},
year = {2023},
date = {2023-04-01},
urldate = {2023-04-01},
journal = {Clin Microbiol Infect},
volume = {29},
number = {4},
pages = {543.e5--543.e9},
abstract = {OBJECTIVES: Our aim was to compare the clinical and virological outcomes in Omicron BA.1- and BA.2-infected patients who received sotrovimab with those in patients who received nirmatrelvir for the prevention of severe COVID-19.nnMETHODS: In this multi-centric, prospective ANRS 0003S CoCoPrev cohort study, patients at a high risk of progression of mild-to-moderate BA.1 or BA.2 COVID-19 who received sotrovimab or nirmatrelvir were included. The proportion of patients with progression to severe COVID-19, time between the start of treatment to negative PCR conversion, SARS-CoV-2 viral decay, and characterization of resistance variants were determined. A multi-variable Cox proportional hazard model was used to determine the time to negative PCR conversion and a mixed-effect model for the dynamics of viral decay.nnRESULTS: Amongst 255 included patients, 199 (80%) received ≥3 vaccine doses, 195 (76%) received sotrovimab, and 60 (24%) received nirmatrelvir. On day 28, new COVID-19-related hospitalization occurred in 4 of 193 (2%; 95% CI, 1-5%) sotrovimab-treated patients and 0 of 55 nirmatrelvir-treated patients (p 0.24). One out of the 55 nirmatrelvir-treated patients died (2%; 95% CI, 0-10%). The median time to negative PCR conversion was 11.5 days (95% CI, 10.5-13) in the sotrovimab-treated patients vs. 4 days (95% CI, 4-9) in the nirmatrelvir-treated patients (p < 0.001). Viral decay was faster in the patients who received nirmatrelvir (p < 0.001). In the multi-variable analysis, nirmatrelvir and nasopharyngeal PCR cycle threshold values were independently associated with faster conversion to negative PCR (hazard ratio, 2.35; 95% CI, 1.56-3.56; p < 0.0001 and hazard ratio, 1.05; 95% CI, 1.01-1.08; p 0.01, respectively).nnCONCLUSIONS: Early administration of nirmatrelvir in high-risk patients compared with that of sotrovimab was associated with faster viral clearance. This may participate to decrease transmission and prevent viral resistance.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
OBJECTIVES: Our aim was to compare the clinical and virological outcomes in Omicron BA.1- and BA.2-infected patients who received sotrovimab with those in patients who received nirmatrelvir for the prevention of severe COVID-19.nnMETHODS: In this multi-centric, prospective ANRS 0003S CoCoPrev cohort study, patients at a high risk of progression of mild-to-moderate BA.1 or BA.2 COVID-19 who received sotrovimab or nirmatrelvir were included. The proportion of patients with progression to severe COVID-19, time between the start of treatment to negative PCR conversion, SARS-CoV-2 viral decay, and characterization of resistance variants were determined. A multi-variable Cox proportional hazard model was used to determine the time to negative PCR conversion and a mixed-effect model for the dynamics of viral decay.nnRESULTS: Amongst 255 included patients, 199 (80%) received ≥3 vaccine doses, 195 (76%) received sotrovimab, and 60 (24%) received nirmatrelvir. On day 28, new COVID-19-related hospitalization occurred in 4 of 193 (2%; 95% CI, 1-5%) sotrovimab-treated patients and 0 of 55 nirmatrelvir-treated patients (p 0.24). One out of the 55 nirmatrelvir-treated patients died (2%; 95% CI, 0-10%). The median time to negative PCR conversion was 11.5 days (95% CI, 10.5-13) in the sotrovimab-treated patients vs. 4 days (95% CI, 4-9) in the nirmatrelvir-treated patients (p < 0.001). Viral decay was faster in the patients who received nirmatrelvir (p < 0.001). In the multi-variable analysis, nirmatrelvir and nasopharyngeal PCR cycle threshold values were independently associated with faster conversion to negative PCR (hazard ratio, 2.35; 95% CI, 1.56-3.56; p < 0.0001 and hazard ratio, 1.05; 95% CI, 1.01-1.08; p 0.01, respectively).nnCONCLUSIONS: Early administration of nirmatrelvir in high-risk patients compared with that of sotrovimab was associated with faster viral clearance. This may participate to decrease transmission and prevent viral resistance. |
Texier, Baptiste; Prime, Morgane; Atamena, Djamaa; Belenguer, Pascale; Szelechowski, Marion Mortalin/Hspa9 involvement and therapeutic perspective in Parkinson's disease Article de journal Dans: Neural Regen Res, vol. 18, no. 2, p. 293–298, 2023, ISSN: 1673-5374. @article{pmid35900406c,
title = {Mortalin/Hspa9 involvement and therapeutic perspective in Parkinson's disease},
author = {Baptiste Texier and Morgane Prime and Djamaa Atamena and Pascale Belenguer and Marion Szelechowski},
doi = {10.4103/1673-5374.346487},
issn = {1673-5374},
year = {2023},
date = {2023-02-01},
urldate = {2023-02-01},
journal = {Neural Regen Res},
volume = {18},
number = {2},
pages = {293--298},
abstract = {By controlling the proper folding of proteins imported into mitochondria and ensuring crosstalk between the reticulum and mitochondria to modulate intracellular calcium fluxes, Mortalin is a chaperone protein that plays crucial roles in neuronal homeostasis and activity. However, its expression and stability are strongly modified in response to cellular stresses, in particular upon altered oxidative conditions during neurodegeneration. Here, we report and discuss the abundant literature that has highlighted its contribution to the pathophysiology of Parkinson's disease, as well as its therapeutic and prognostic potential in this still incurable pathology.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
By controlling the proper folding of proteins imported into mitochondria and ensuring crosstalk between the reticulum and mitochondria to modulate intracellular calcium fluxes, Mortalin is a chaperone protein that plays crucial roles in neuronal homeostasis and activity. However, its expression and stability are strongly modified in response to cellular stresses, in particular upon altered oxidative conditions during neurodegeneration. Here, we report and discuss the abundant literature that has highlighted its contribution to the pathophysiology of Parkinson's disease, as well as its therapeutic and prognostic potential in this still incurable pathology. |
Faure, Fabrice; Yshii, Lidia; Renno, Toufic; Coste, Isabelle; Joubert, Bastien; Desestret, Virginie; Liblau, Roland; Honnorat, Jérôme A Pilot Study to Develop Paraneoplastic Cerebellar Degeneration Mouse Model Article de journal Dans: Cerebellum, 2023, ISSN: 1473-4230. @article{pmid36729270,
title = {A Pilot Study to Develop Paraneoplastic Cerebellar Degeneration Mouse Model},
author = {Fabrice Faure and Lidia Yshii and Toufic Renno and Isabelle Coste and Bastien Joubert and Virginie Desestret and Roland Liblau and Jérôme Honnorat},
doi = {10.1007/s12311-023-01524-6},
issn = {1473-4230},
year = {2023},
date = {2023-02-01},
urldate = {2023-02-01},
journal = {Cerebellum},
abstract = {Modeling paraneoplastic neurological diseases to understand the immune mechanisms leading to neuronal death is a major challenge given the rarity and terminal access of patients' autopsies. Here, we present a pilot study aiming at modeling paraneoplastic cerebellar degeneration with Yo autoantibodies (Yo-PCD). Female mice were implanted with an ovarian carcinoma cell line expressing CDR2 and CDR2L, the known antigens recognized by anti-Yo antibodies. To boost the immune response, we also immunized the mice by injecting antigens with diverse adjuvants and immune checkpoint inhibitors. Ataxia and gait instability were assessed in treated mice as well as autoantibody levels, Purkinje cell density, and immune infiltration in the cerebellum. We observed the production of anti-Yo antibodies in the CSF and serum of all immunized mice. Brain immunoreaction varied depending on the site of implantation of the tumor, with subcutaneous administration leading to a massive infiltration of immune cells in the meningeal spaces, choroid plexus, and cerebellar parenchyma. However, we did not observe massive Purkinje cell death nor any motor impairments in any of the experimental groups. Self-sustained neuro-inflammation might require a longer time to build up in our model. Unusual tumor antigen presentation and/or intrinsic, species-specific factors required for pro-inflammatory engagement in the brain may also constitute strong limitations to achieve massive recruitment of antigen-specific T-cells and killing of antigen-expressing neurons in this mouse model.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Modeling paraneoplastic neurological diseases to understand the immune mechanisms leading to neuronal death is a major challenge given the rarity and terminal access of patients' autopsies. Here, we present a pilot study aiming at modeling paraneoplastic cerebellar degeneration with Yo autoantibodies (Yo-PCD). Female mice were implanted with an ovarian carcinoma cell line expressing CDR2 and CDR2L, the known antigens recognized by anti-Yo antibodies. To boost the immune response, we also immunized the mice by injecting antigens with diverse adjuvants and immune checkpoint inhibitors. Ataxia and gait instability were assessed in treated mice as well as autoantibody levels, Purkinje cell density, and immune infiltration in the cerebellum. We observed the production of anti-Yo antibodies in the CSF and serum of all immunized mice. Brain immunoreaction varied depending on the site of implantation of the tumor, with subcutaneous administration leading to a massive infiltration of immune cells in the meningeal spaces, choroid plexus, and cerebellar parenchyma. However, we did not observe massive Purkinje cell death nor any motor impairments in any of the experimental groups. Self-sustained neuro-inflammation might require a longer time to build up in our model. Unusual tumor antigen presentation and/or intrinsic, species-specific factors required for pro-inflammatory engagement in the brain may also constitute strong limitations to achieve massive recruitment of antigen-specific T-cells and killing of antigen-expressing neurons in this mouse model. |
Astier, Anne L; Kofler, David M Editorial: Dysregulation of Th17 and Treg cells in autoimmune diseases Article de journal Dans: Front Immunol, vol. 14, p. 1151836, 2023, ISSN: 1664-3224. @article{pmid36865563,
title = {Editorial: Dysregulation of Th17 and Treg cells in autoimmune diseases},
author = {Anne L Astier and David M Kofler},
doi = {10.3389/fimmu.2023.1151836},
issn = {1664-3224},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {Front Immunol},
volume = {14},
pages = {1151836},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
de Sèze, Jérôme; Maillart, Elisabeth; Gueguen, Antoine; Laplaud, David A; Michel, Laure; Thouvenot, Eric; Zephir, Hélène; Zimmer, Luc; Biotti, Damien; Liblau, Roland Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic Article de journal Dans: Front Immunol, vol. 14, p. 1004795, 2023, ISSN: 1664-3224. @article{pmid37033984,
title = {Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic},
author = {Jérôme de Sèze and Elisabeth Maillart and Antoine Gueguen and David A Laplaud and Laure Michel and Eric Thouvenot and Hélène Zephir and Luc Zimmer and Damien Biotti and Roland Liblau},
doi = {10.3389/fimmu.2023.1004795},
issn = {1664-3224},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {Front Immunol},
volume = {14},
pages = {1004795},
abstract = {The immune system plays a significant role in multiple sclerosis. While MS was historically thought to be T cell-mediated, multiple pieces of evidence now support the view that B cells are essential players in multiple sclerosis pathogenic processes. High-efficacy disease-modifying therapies that target the immune system have emerged over the past two decades. Anti-CD20 monoclonal antibodies selectively deplete CD20+ B and CD20+ T cells and efficiently suppress inflammatory disease activity. These monotherapies prevent relapses, reduce new or active magnetic resonance imaging brain lesions, and lessen disability progression in patients with relapsing multiple sclerosis. Rituximab, ocrelizumab, and ofatumumab are currently used in clinical practice, while phase III clinical trials for ublituximab have been recently completed. In this review, we compare the four anti-CD20 antibodies in terms of their mechanisms of action, routes of administration, immunological targets, and pharmacokinetic properties. A deeper understanding of the individual properties of these molecules in relation to their efficacy and safety profiles is critical for their use in clinical practice.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The immune system plays a significant role in multiple sclerosis. While MS was historically thought to be T cell-mediated, multiple pieces of evidence now support the view that B cells are essential players in multiple sclerosis pathogenic processes. High-efficacy disease-modifying therapies that target the immune system have emerged over the past two decades. Anti-CD20 monoclonal antibodies selectively deplete CD20+ B and CD20+ T cells and efficiently suppress inflammatory disease activity. These monotherapies prevent relapses, reduce new or active magnetic resonance imaging brain lesions, and lessen disability progression in patients with relapsing multiple sclerosis. Rituximab, ocrelizumab, and ofatumumab are currently used in clinical practice, while phase III clinical trials for ublituximab have been recently completed. In this review, we compare the four anti-CD20 antibodies in terms of their mechanisms of action, routes of administration, immunological targets, and pharmacokinetic properties. A deeper understanding of the individual properties of these molecules in relation to their efficacy and safety profiles is critical for their use in clinical practice. |
Ayoub, Ikram; Dauvilliers, Yves; Barateau, Lucie; Vermeulen, Thaïs; Mouton-Barbosa, Emmanuelle; Marcellin, Marlène; Gonzalez-de-Peredo, Anne; Gross, Catharina C; Saoudi, Abdelhadi; Liblau, Roland Cerebrospinal fluid proteomics in recent-onset Narcolepsy type 1 reveals activation of the complement system Article de journal Dans: Front Immunol, vol. 14, p. 1108682, 2023, ISSN: 1664-3224. @article{pmid37122721,
title = {Cerebrospinal fluid proteomics in recent-onset Narcolepsy type 1 reveals activation of the complement system},
author = {Ikram Ayoub and Yves Dauvilliers and Lucie Barateau and Thaïs Vermeulen and Emmanuelle Mouton-Barbosa and Marlène Marcellin and Anne Gonzalez-de-Peredo and Catharina C Gross and Abdelhadi Saoudi and Roland Liblau},
doi = {10.3389/fimmu.2023.1108682},
issn = {1664-3224},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {Front Immunol},
volume = {14},
pages = {1108682},
abstract = {INTRODUCTION: Narcolepsy type 1 (NT1) is a rare, chronic and disabling neurological disease causing excessive daytime sleepiness and cataplexy. NT1 is characterized pathologically by an almost complete loss of neurons producing the orexin neuropeptides in the lateral hypothalamus. Genetic and environmental factors strongly suggest the involvement of the immune system in the loss of orexin neurons. The cerebrospinal fluid (CSF), secreted locally and surrounding the central nervous system (CNS), represents an accessible window into CNS pathological processes.nnMETHODS: To gain insight into the biological and molecular changes in NT1 patients, we performed a comparative proteomics analysis of the CSF from 21 recent-onset NT1 patients and from two control groups: group 1 with somatoform disorders, and group 2 patients with hypersomnia other than NT1, to control for any potential effect of sleep disturbances on CSF composition. To achieve an optimal proteomic coverage analysis, the twelve most abundant CSF proteins were depleted, and samples were analyzed by nano-flow liquid chromatography tandem mass spectrometry (nano-LC-MS/MS) using the latest generation of hybrid Orbitrap mass spectrometer.nnRESULTS AND DISCUSSION: Our study allowed the identification and quantification of up to 1943 proteins, providing a remarkably deep analysis of the CSF proteome. Interestingly, gene set enrichment analysis indicated that the complement and coagulation systems were enriched and significantly activated in NT1 patients in both cohorts analyzed. Notably, the lectin and alternative complement pathway as well as the downstream lytic membrane attack complex were congruently increased in NT1. Our data suggest that the complement dysregulation in NT1 patients can contribute to immunopathology either by directly promoting tissue damage or as part of local inflammatory responses. We therefore reveal an altered composition of the CSF proteome in NT1 patients, which points to an ongoing inflammatory process contributed, at least in part, by the complement system.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
INTRODUCTION: Narcolepsy type 1 (NT1) is a rare, chronic and disabling neurological disease causing excessive daytime sleepiness and cataplexy. NT1 is characterized pathologically by an almost complete loss of neurons producing the orexin neuropeptides in the lateral hypothalamus. Genetic and environmental factors strongly suggest the involvement of the immune system in the loss of orexin neurons. The cerebrospinal fluid (CSF), secreted locally and surrounding the central nervous system (CNS), represents an accessible window into CNS pathological processes.nnMETHODS: To gain insight into the biological and molecular changes in NT1 patients, we performed a comparative proteomics analysis of the CSF from 21 recent-onset NT1 patients and from two control groups: group 1 with somatoform disorders, and group 2 patients with hypersomnia other than NT1, to control for any potential effect of sleep disturbances on CSF composition. To achieve an optimal proteomic coverage analysis, the twelve most abundant CSF proteins were depleted, and samples were analyzed by nano-flow liquid chromatography tandem mass spectrometry (nano-LC-MS/MS) using the latest generation of hybrid Orbitrap mass spectrometer.nnRESULTS AND DISCUSSION: Our study allowed the identification and quantification of up to 1943 proteins, providing a remarkably deep analysis of the CSF proteome. Interestingly, gene set enrichment analysis indicated that the complement and coagulation systems were enriched and significantly activated in NT1 patients in both cohorts analyzed. Notably, the lectin and alternative complement pathway as well as the downstream lytic membrane attack complex were congruently increased in NT1. Our data suggest that the complement dysregulation in NT1 patients can contribute to immunopathology either by directly promoting tissue damage or as part of local inflammatory responses. We therefore reveal an altered composition of the CSF proteome in NT1 patients, which points to an ongoing inflammatory process contributed, at least in part, by the complement system. |
2022
|
Schneider-Hohendorf, Tilman; Gerdes, Lisa Ann; Pignolet, Béatrice; Gittelman, Rachel; Ostkamp, Patrick; Rubelt, Florian; Raposo, Catarina; Tackenberg, Björn; Riepenhausen, Marianne; Janoschka, Claudia; Wünsch, Christian; Bucciarelli, Florence; Flierl-Hecht, Andrea; Beltrán, Eduardo; Kümpfel, Tania; Anslinger, Katja; Gross, Catharina C; Chapman, Heidi; Kaplan, Ian; Brassat, David; Wekerle, Hartmut; Kerschensteiner, Martin; Klotz, Luisa; Lünemann, Jan D; Hohlfeld, Reinhard; Liblau, Roland; Wiendl, Heinz; Schwab, Nicholas Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis Article de journal Dans: J Exp Med, vol. 219, no. 11, 2022, ISSN: 1540-9538. @article{pmid36048016,
title = {Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis},
author = {Tilman Schneider-Hohendorf and Lisa Ann Gerdes and Béatrice Pignolet and Rachel Gittelman and Patrick Ostkamp and Florian Rubelt and Catarina Raposo and Björn Tackenberg and Marianne Riepenhausen and Claudia Janoschka and Christian Wünsch and Florence Bucciarelli and Andrea Flierl-Hecht and Eduardo Beltrán and Tania Kümpfel and Katja Anslinger and Catharina C Gross and Heidi Chapman and Ian Kaplan and David Brassat and Hartmut Wekerle and Martin Kerschensteiner and Luisa Klotz and Jan D Lünemann and Reinhard Hohlfeld and Roland Liblau and Heinz Wiendl and Nicholas Schwab},
doi = {10.1084/jem.20220650},
issn = {1540-9538},
year = {2022},
date = {2022-11-01},
urldate = {2022-11-01},
journal = {J Exp Med},
volume = {219},
number = {11},
abstract = {Epstein-Barr virus (EBV) infection precedes multiple sclerosis (MS) pathology and cross-reactive antibodies might link EBV infection to CNS autoimmunity. As an altered anti-EBV T cell reaction was suggested in MS, we queried peripheral blood T cell receptor β chain (TCRβ) repertoires of 1,395 MS patients, 887 controls, and 35 monozygotic, MS-discordant twin pairs for multimer-confirmed, viral antigen-specific TCRβ sequences. We detected more MHC-I-restricted EBV-specific TCRβ sequences in MS patients. Differences in genetics or upbringing could be excluded by validation in monozygotic twin pairs discordant for MS. Anti-VLA-4 treatment amplified this observation, while interferon β- or anti-CD20 treatment did not modulate EBV-specific T cell occurrence. In healthy individuals, EBV-specific CD8+ T cells were of an effector-memory phenotype in peripheral blood and cerebrospinal fluid. In MS patients, cerebrospinal fluid also contained EBV-specific central-memory CD8+ T cells, suggesting recent priming. Therefore, MS is not only preceded by EBV infection, but also associated with broader EBV-specific TCR repertoires, consistent with an ongoing anti-EBV immune reaction in MS.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Epstein-Barr virus (EBV) infection precedes multiple sclerosis (MS) pathology and cross-reactive antibodies might link EBV infection to CNS autoimmunity. As an altered anti-EBV T cell reaction was suggested in MS, we queried peripheral blood T cell receptor β chain (TCRβ) repertoires of 1,395 MS patients, 887 controls, and 35 monozygotic, MS-discordant twin pairs for multimer-confirmed, viral antigen-specific TCRβ sequences. We detected more MHC-I-restricted EBV-specific TCRβ sequences in MS patients. Differences in genetics or upbringing could be excluded by validation in monozygotic twin pairs discordant for MS. Anti-VLA-4 treatment amplified this observation, while interferon β- or anti-CD20 treatment did not modulate EBV-specific T cell occurrence. In healthy individuals, EBV-specific CD8+ T cells were of an effector-memory phenotype in peripheral blood and cerebrospinal fluid. In MS patients, cerebrospinal fluid also contained EBV-specific central-memory CD8+ T cells, suggesting recent priming. Therefore, MS is not only preceded by EBV infection, but also associated with broader EBV-specific TCR repertoires, consistent with an ongoing anti-EBV immune reaction in MS. |
Martin-Blondel, Guillaume; Marcelin, Anne-Genevieve; Soulié, Cathia; Kaisaridi, Sofia; Lusivika-Nzinga, Clovis; Dorival, Céline; Nailler, Laura; Boston, Anaïs; Melenotte, Cléa; Cabié, André; Choquet, Christophe; Coustillères, François; Martellosio, Jean-Philippe; Gaube, Géraldine; Trinh-Duc, Albert; Ronchetti, Anne-Marie; Pourcher, Valerie; Chauveau, Marie; Lacombe, Karine; Peiffer-Smadja, Nathan; Housset, Pierre; Perrot, Aurore; Pialoux, Gilles; Martin, Aurélie; Dubee, Vincent; Devaux, Mathilde; Frey, Jérôme; Cazanave, Charles; Liblau, Roland; Carrat, Fabrice; Yordanov, Youri Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2 Divers 2022, ISSN: 1532-2742. @misc{pmid35803386,
title = {Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2},
author = {Guillaume Martin-Blondel and Anne-Genevieve Marcelin and Cathia Soulié and Sofia Kaisaridi and Clovis Lusivika-Nzinga and Céline Dorival and Laura Nailler and Anaïs Boston and Cléa Melenotte and André Cabié and Christophe Choquet and François Coustillères and Jean-Philippe Martellosio and Géraldine Gaube and Albert Trinh-Duc and Anne-Marie Ronchetti and Valerie Pourcher and Marie Chauveau and Karine Lacombe and Nathan Peiffer-Smadja and Pierre Housset and Aurore Perrot and Gilles Pialoux and Aurélie Martin and Vincent Dubee and Mathilde Devaux and Jérôme Frey and Charles Cazanave and Roland Liblau and Fabrice Carrat and Youri Yordanov},
doi = {10.1016/j.jinf.2022.06.033},
issn = {1532-2742},
year = {2022},
date = {2022-10-01},
urldate = {2022-10-01},
journal = {J Infect},
volume = {85},
number = {4},
pages = {e104--e108},
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
|
Chiu, Isaac M; Liblau, Roland Editorial overview: Special section neuroimmunology: Neuroimmune interactions in health and disease Divers 2022, ISSN: 1879-0372. @misc{pmid35792467,
title = {Editorial overview: Special section neuroimmunology: Neuroimmune interactions in health and disease},
author = {Isaac M Chiu and Roland Liblau},
doi = {10.1016/j.coi.2022.102232},
issn = {1879-0372},
year = {2022},
date = {2022-08-01},
urldate = {2022-08-01},
journal = {Curr Opin Immunol},
volume = {77},
pages = {102232},
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
|
Merkler, Doron; Vincenti, Ilena; Masson, Frederick; Liblau, Roland S Tissue-resident CD8 T cells in central nervous system inflammatory diseases: present at the crime scene and …guilty Article de journal Dans: Curr Opin Immunol, vol. 77, p. 102211, 2022, ISSN: 1879-0372. @article{pmid35644112,
title = {Tissue-resident CD8 T cells in central nervous system inflammatory diseases: present at the crime scene and …guilty},
author = {Doron Merkler and Ilena Vincenti and Frederick Masson and Roland S Liblau},
doi = {10.1016/j.coi.2022.102211},
issn = {1879-0372},
year = {2022},
date = {2022-08-01},
urldate = {2022-08-01},
journal = {Curr Opin Immunol},
volume = {77},
pages = {102211},
abstract = {Tissue-resident memory T cells (T) represent a subset of antigen-experienced T cells that are constantly retained in a given tissue with limited trafficking through the circulation. These cells are characterized by expression of molecules enabling their tissue anchoring and downregulation of molecules promoting tissue egress. They reside at sites of previous antigen encounter and their number increases with age. T have been shown to provide rapid and efficient protection against tissue reinfection and T density correlates with efficient antitumor responses. Intriguingly, the density of CD8 T is increased in the central nervous system (CNS) of patients with neuroinflammatory diseases such as multiple sclerosis, or suffering from neurodegenerative diseases. In this review, we discuss current knowledge regarding the diversity of CNS-resident CD8 T cells and their role in CNS autoimmunity. Given their likely contribution to the protracted course of several inflammatory diseases of the CNS, their therapeutic targeting becomes an important challenge.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Tissue-resident memory T cells (T) represent a subset of antigen-experienced T cells that are constantly retained in a given tissue with limited trafficking through the circulation. These cells are characterized by expression of molecules enabling their tissue anchoring and downregulation of molecules promoting tissue egress. They reside at sites of previous antigen encounter and their number increases with age. T have been shown to provide rapid and efficient protection against tissue reinfection and T density correlates with efficient antitumor responses. Intriguingly, the density of CD8 T is increased in the central nervous system (CNS) of patients with neuroinflammatory diseases such as multiple sclerosis, or suffering from neurodegenerative diseases. In this review, we discuss current knowledge regarding the diversity of CNS-resident CD8 T cells and their role in CNS autoimmunity. Given their likely contribution to the protracted course of several inflammatory diseases of the CNS, their therapeutic targeting becomes an important challenge. |
Bernard-Valnet, Raphaël; Frieser, David; Nguyen, Xuan Hung; Khajavi, Leila; Quériault, Clémence; Arthaud, Sébastien; Melzi, Silvia; Fusade-Boyer, Maxime; Masson, Frederick; Zytnicki, Matthias; Saoudi, Abdelhadi; Dauvilliers, Yves; Peyron, Christelle; Bauer, Jan; Liblau, Roland S Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy Article de journal Dans: Brain, vol. 145, no. 6, p. 2018–2030, 2022, ISSN: 1460-2156. @article{pmid35552381b,
title = {Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy},
author = {Raphaël Bernard-Valnet and David Frieser and Xuan Hung Nguyen and Leila Khajavi and Clémence Quériault and Sébastien Arthaud and Silvia Melzi and Maxime Fusade-Boyer and Frederick Masson and Matthias Zytnicki and Abdelhadi Saoudi and Yves Dauvilliers and Christelle Peyron and Jan Bauer and Roland S Liblau},
doi = {10.1093/brain/awab455},
issn = {1460-2156},
year = {2022},
date = {2022-06-01},
urldate = {2022-06-01},
journal = {Brain},
volume = {145},
number = {6},
pages = {2018--2030},
abstract = {Narcolepsy with cataplexy or narcolepsy type 1 is a disabling chronic sleep disorder resulting from the destruction of orexinergic neurons in the hypothalamus. The tight association of narcolepsy with HLA-DQB1*06:02 strongly suggest an autoimmune origin to this disease. Furthermore, converging epidemiological studies have identified an increased incidence for narcolepsy in Europe following Pandemrix® vaccination against the 2009-2010 pandemic 'influenza' virus strain. The potential immunological link between the Pandemrix® vaccination and narcolepsy remains, however, unknown. Deciphering these mechanisms may reveal pathways potentially at play in most cases of narcolepsy. Here, we developed a mouse model allowing to track and study the T-cell response against 'influenza' virus haemagglutinin, which was selectively expressed in the orexinergic neurons as a new self-antigen. Pandemrix® vaccination in this mouse model resulted in hypothalamic inflammation and selective destruction of orexin-producing neurons. Further investigations on the relative contribution of T-cell subsets in this process revealed that haemagglutinin-specific CD4 T cells were necessary for the development of hypothalamic inflammation, but insufficient for killing orexinergic neurons. Conversely, haemagglutinin-specific CD8 T cells could not initiate inflammation but were the effectors of the destruction of orexinergic neurons. Additional studies revealed pathways potentially involved in the disease process. Notably, the interferon-γ pathway was proven essential, as interferon-γ-deficient CD8 T cells were unable to elicit the loss of orexinergic neurons. Our work demonstrates that an immunopathological process mimicking narcolepsy can be elicited by immune cross-reactivity between a vaccine antigen and a neuronal self-antigen. This process relies on a synergy between autoreactive CD4 and CD8 T cells for disease development. This work furthers our understanding of the mechanisms and pathways potentially involved in the development of a neurological side effect due to a vaccine and, likely, to narcolepsy in general.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Narcolepsy with cataplexy or narcolepsy type 1 is a disabling chronic sleep disorder resulting from the destruction of orexinergic neurons in the hypothalamus. The tight association of narcolepsy with HLA-DQB1*06:02 strongly suggest an autoimmune origin to this disease. Furthermore, converging epidemiological studies have identified an increased incidence for narcolepsy in Europe following Pandemrix® vaccination against the 2009-2010 pandemic 'influenza' virus strain. The potential immunological link between the Pandemrix® vaccination and narcolepsy remains, however, unknown. Deciphering these mechanisms may reveal pathways potentially at play in most cases of narcolepsy. Here, we developed a mouse model allowing to track and study the T-cell response against 'influenza' virus haemagglutinin, which was selectively expressed in the orexinergic neurons as a new self-antigen. Pandemrix® vaccination in this mouse model resulted in hypothalamic inflammation and selective destruction of orexin-producing neurons. Further investigations on the relative contribution of T-cell subsets in this process revealed that haemagglutinin-specific CD4 T cells were necessary for the development of hypothalamic inflammation, but insufficient for killing orexinergic neurons. Conversely, haemagglutinin-specific CD8 T cells could not initiate inflammation but were the effectors of the destruction of orexinergic neurons. Additional studies revealed pathways potentially involved in the disease process. Notably, the interferon-γ pathway was proven essential, as interferon-γ-deficient CD8 T cells were unable to elicit the loss of orexinergic neurons. Our work demonstrates that an immunopathological process mimicking narcolepsy can be elicited by immune cross-reactivity between a vaccine antigen and a neuronal self-antigen. This process relies on a synergy between autoreactive CD4 and CD8 T cells for disease development. This work furthers our understanding of the mechanisms and pathways potentially involved in the development of a neurological side effect due to a vaccine and, likely, to narcolepsy in general. |
Bernard-Valnet, Raphaël; Frieser, David; Nguyen, Xuan Hung; Khajavi, Leila; Quériault, Clémence; Arthaud, Sébastien; Melzi, Silvia; Fusade-Boyer, Maxime; Masson, Frederick; Zytnicki, Matthias; Saoudi, Abdelhadi; Dauvilliers, Yves; Peyron, Christelle; Bauer, Jan; Liblau, Roland S Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy Article de journal Dans: Brain, vol. 145, no. 6, p. 2018–2030, 2022, ISSN: 1460-2156. @article{pmid35552381,
title = {Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy},
author = {Raphaël Bernard-Valnet and David Frieser and Xuan Hung Nguyen and Leila Khajavi and Clémence Quériault and Sébastien Arthaud and Silvia Melzi and Maxime Fusade-Boyer and Frederick Masson and Matthias Zytnicki and Abdelhadi Saoudi and Yves Dauvilliers and Christelle Peyron and Jan Bauer and Roland S Liblau},
doi = {10.1093/brain/awab455},
issn = {1460-2156},
year = {2022},
date = {2022-06-01},
urldate = {2022-06-01},
journal = {Brain},
volume = {145},
number = {6},
pages = {2018--2030},
abstract = {Narcolepsy with cataplexy or narcolepsy type 1 is a disabling chronic sleep disorder resulting from the destruction of orexinergic neurons in the hypothalamus. The tight association of narcolepsy with HLA-DQB1*06:02 strongly suggest an autoimmune origin to this disease. Furthermore, converging epidemiological studies have identified an increased incidence for narcolepsy in Europe following Pandemrix® vaccination against the 2009-2010 pandemic 'influenza' virus strain. The potential immunological link between the Pandemrix® vaccination and narcolepsy remains, however, unknown. Deciphering these mechanisms may reveal pathways potentially at play in most cases of narcolepsy. Here, we developed a mouse model allowing to track and study the T-cell response against 'influenza' virus haemagglutinin, which was selectively expressed in the orexinergic neurons as a new self-antigen. Pandemrix® vaccination in this mouse model resulted in hypothalamic inflammation and selective destruction of orexin-producing neurons. Further investigations on the relative contribution of T-cell subsets in this process revealed that haemagglutinin-specific CD4 T cells were necessary for the development of hypothalamic inflammation, but insufficient for killing orexinergic neurons. Conversely, haemagglutinin-specific CD8 T cells could not initiate inflammation but were the effectors of the destruction of orexinergic neurons. Additional studies revealed pathways potentially involved in the disease process. Notably, the interferon-γ pathway was proven essential, as interferon-γ-deficient CD8 T cells were unable to elicit the loss of orexinergic neurons. Our work demonstrates that an immunopathological process mimicking narcolepsy can be elicited by immune cross-reactivity between a vaccine antigen and a neuronal self-antigen. This process relies on a synergy between autoreactive CD4 and CD8 T cells for disease development. This work furthers our understanding of the mechanisms and pathways potentially involved in the development of a neurological side effect due to a vaccine and, likely, to narcolepsy in general.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Narcolepsy with cataplexy or narcolepsy type 1 is a disabling chronic sleep disorder resulting from the destruction of orexinergic neurons in the hypothalamus. The tight association of narcolepsy with HLA-DQB1*06:02 strongly suggest an autoimmune origin to this disease. Furthermore, converging epidemiological studies have identified an increased incidence for narcolepsy in Europe following Pandemrix® vaccination against the 2009-2010 pandemic 'influenza' virus strain. The potential immunological link between the Pandemrix® vaccination and narcolepsy remains, however, unknown. Deciphering these mechanisms may reveal pathways potentially at play in most cases of narcolepsy. Here, we developed a mouse model allowing to track and study the T-cell response against 'influenza' virus haemagglutinin, which was selectively expressed in the orexinergic neurons as a new self-antigen. Pandemrix® vaccination in this mouse model resulted in hypothalamic inflammation and selective destruction of orexin-producing neurons. Further investigations on the relative contribution of T-cell subsets in this process revealed that haemagglutinin-specific CD4 T cells were necessary for the development of hypothalamic inflammation, but insufficient for killing orexinergic neurons. Conversely, haemagglutinin-specific CD8 T cells could not initiate inflammation but were the effectors of the destruction of orexinergic neurons. Additional studies revealed pathways potentially involved in the disease process. Notably, the interferon-γ pathway was proven essential, as interferon-γ-deficient CD8 T cells were unable to elicit the loss of orexinergic neurons. Our work demonstrates that an immunopathological process mimicking narcolepsy can be elicited by immune cross-reactivity between a vaccine antigen and a neuronal self-antigen. This process relies on a synergy between autoreactive CD4 and CD8 T cells for disease development. This work furthers our understanding of the mechanisms and pathways potentially involved in the development of a neurological side effect due to a vaccine and, likely, to narcolepsy in general. |
Gaudenzio, Nicolas; Liblau, Roland S Immune cells impede repair of old neurons Article de journal Dans: Science, vol. 376, no. 6594, p. 694–695, 2022, ISSN: 1095-9203. @article{pmid35549427,
title = {Immune cells impede repair of old neurons},
author = {Nicolas Gaudenzio and Roland S Liblau},
doi = {10.1126/science.abp9878},
issn = {1095-9203},
year = {2022},
date = {2022-05-01},
urldate = {2022-05-01},
journal = {Science},
volume = {376},
number = {6594},
pages = {694--695},
abstract = {Interfering with age-related neuroimmune interactions promotes nerve regeneration.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Interfering with age-related neuroimmune interactions promotes nerve regeneration. |
Frieser, David; Pignata, Aurora; Khajavi, Leila; Shlesinger, Danielle; Gonzalez-Fierro, Carmen; Nguyen, Xuan-Hung; Yermanos, Alexander; Merkler, Doron; Höftberger, Romana; Desestret, Virginie; Mair, Katharina M; Bauer, Jan; Masson, Frederick; Liblau, Roland S Tissue-resident CD8 T cells drive compartmentalized and chronic autoimmune damage against CNS neurons Article de journal Dans: Sci Transl Med, vol. 14, no. 640, p. eabl6157, 2022, ISSN: 1946-6242. @article{pmid35417189,
title = {Tissue-resident CD8 T cells drive compartmentalized and chronic autoimmune damage against CNS neurons},
author = {David Frieser and Aurora Pignata and Leila Khajavi and Danielle Shlesinger and Carmen Gonzalez-Fierro and Xuan-Hung Nguyen and Alexander Yermanos and Doron Merkler and Romana Höftberger and Virginie Desestret and Katharina M Mair and Jan Bauer and Frederick Masson and Roland S Liblau},
doi = {10.1126/scitranslmed.abl6157},
issn = {1946-6242},
year = {2022},
date = {2022-04-01},
urldate = {2022-04-01},
journal = {Sci Transl Med},
volume = {14},
number = {640},
pages = {eabl6157},
abstract = {The mechanisms underlying the chronicity of autoimmune diseases of the central nervous system (CNS) are largely unknown. In particular, it is unclear whether tissue-resident memory T cells (T) contribute to lesion pathogenesis during chronic CNS autoimmunity. Here, we observed that a high frequency of brain-infiltrating CD8 T cells exhibit a T-like phenotype in human autoimmune encephalitis. Using mouse models of neuronal autoimmunity and a combination of T single-cell transcriptomics, high-dimensional flow cytometry, and histopathology, we found that pathogenic CD8 T cells behind the blood-brain barrier adopt a characteristic T differentiation program, and we revealed their phenotypic and functional heterogeneity. In the diseased CNS, autoreactive tissue-resident CD8 T cells sustained focal neuroinflammation and progressive loss of neurons, independently of recirculating CD8 T cells. Consistently, a large fraction of autoreactive tissue-resident CD8 T cells exhibited proliferative potential as well as proinflammatory and cytotoxic properties. Persistence of tissue-resident CD8 T cells in the CNS and their functional output, but not their initial differentiation, were crucially dependent on CD4 T cells. Collectively, our results point to tissue-resident CD8 T cells as essential drivers of chronic CNS autoimmunity and suggest that therapies targeting this compartmentalized autoreactive T cell subset might be effective for treating CNS autoimmune diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The mechanisms underlying the chronicity of autoimmune diseases of the central nervous system (CNS) are largely unknown. In particular, it is unclear whether tissue-resident memory T cells (T) contribute to lesion pathogenesis during chronic CNS autoimmunity. Here, we observed that a high frequency of brain-infiltrating CD8 T cells exhibit a T-like phenotype in human autoimmune encephalitis. Using mouse models of neuronal autoimmunity and a combination of T single-cell transcriptomics, high-dimensional flow cytometry, and histopathology, we found that pathogenic CD8 T cells behind the blood-brain barrier adopt a characteristic T differentiation program, and we revealed their phenotypic and functional heterogeneity. In the diseased CNS, autoreactive tissue-resident CD8 T cells sustained focal neuroinflammation and progressive loss of neurons, independently of recirculating CD8 T cells. Consistently, a large fraction of autoreactive tissue-resident CD8 T cells exhibited proliferative potential as well as proinflammatory and cytotoxic properties. Persistence of tissue-resident CD8 T cells in the CNS and their functional output, but not their initial differentiation, were crucially dependent on CD4 T cells. Collectively, our results point to tissue-resident CD8 T cells as essential drivers of chronic CNS autoimmunity and suggest that therapies targeting this compartmentalized autoreactive T cell subset might be effective for treating CNS autoimmune diseases. |
Hohlfeld, Reinhard; Liblau, Roland S Toward identification of personalized immunological profiles in multiple sclerosis Article de journal Dans: Sci Adv, vol. 8, no. 17, p. eabq4849, 2022, ISSN: 2375-2548. @article{pmid35476442,
title = {Toward identification of personalized immunological profiles in multiple sclerosis},
author = {Reinhard Hohlfeld and Roland S Liblau},
doi = {10.1126/sciadv.abq4849},
issn = {2375-2548},
year = {2022},
date = {2022-04-01},
urldate = {2022-04-01},
journal = {Sci Adv},
volume = {8},
number = {17},
pages = {eabq4849},
abstract = {The diversity of four previously unidentified autoantigens found in multiple sclerosis mirrors its notorious clinical variability.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The diversity of four previously unidentified autoantigens found in multiple sclerosis mirrors its notorious clinical variability. |
Kreutmair, Stefanie; Unger, Susanne; Núñez, Nicolás Gonzalo; Ingelfinger, Florian; Alberti, Chiara; Feo, Donatella De; Krishnarajah, Sinduya; Kauffmann, Manuel; Friebel, Ekaterina; Babaei, Sepideh; Gaborit, Benjamin; Lutz, Mirjam; Jurado, Nicole Puertas; Malek, Nisar P; Goepel, Siri; Rosenberger, Peter; Häberle, Helene A; Ayoub, Ikram; Al-Hajj, Sally; Nilsson, Jakob; Claassen, Manfred; Liblau, Roland; Martin-Blondel, Guillaume; Bitzer, Michael; Roquilly, Antoine; Becher, Burkhard Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia Divers 2022, ISSN: 1097-4180. @misc{pmid35139354,
title = {Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia},
author = {Stefanie Kreutmair and Susanne Unger and Nicolás Gonzalo Núñez and Florian Ingelfinger and Chiara Alberti and Donatella De Feo and Sinduya Krishnarajah and Manuel Kauffmann and Ekaterina Friebel and Sepideh Babaei and Benjamin Gaborit and Mirjam Lutz and Nicole Puertas Jurado and Nisar P Malek and Siri Goepel and Peter Rosenberger and Helene A Häberle and Ikram Ayoub and Sally Al-Hajj and Jakob Nilsson and Manfred Claassen and Roland Liblau and Guillaume Martin-Blondel and Michael Bitzer and Antoine Roquilly and Burkhard Becher},
doi = {10.1016/j.immuni.2022.01.015},
issn = {1097-4180},
year = {2022},
date = {2022-02-01},
urldate = {2022-02-01},
journal = {Immunity},
volume = {55},
number = {2},
pages = {366--375},
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
|
Galoppin, Manon; Kari, Saniya; Soldati, Sasha; Pal, Arindam; Rival, Manon; Engelhardt, Britta; Astier, Anne; Thouvenot, Eric Full spectrum of vitamin D immunomodulation in multiple sclerosis: mechanisms and therapeutic implications Article de journal Dans: Brain Commun, vol. 4, no. 4, p. fcac171, 2022, ISSN: 2632-1297. @article{pmid35813882,
title = {Full spectrum of vitamin D immunomodulation in multiple sclerosis: mechanisms and therapeutic implications},
author = {Manon Galoppin and Saniya Kari and Sasha Soldati and Arindam Pal and Manon Rival and Britta Engelhardt and Anne Astier and Eric Thouvenot},
doi = {10.1093/braincomms/fcac171},
issn = {2632-1297},
year = {2022},
date = {2022-01-01},
urldate = {2022-01-01},
journal = {Brain Commun},
volume = {4},
number = {4},
pages = {fcac171},
abstract = {Vitamin D deficiency has been associated with the risk of multiple sclerosis, disease activity and progression. Results from experiments, animal models and analysis of human samples from randomized controlled trials provide comprehensive data illustrating the pleiotropic actions of Vitamin D on the immune system. They globally result in immunomodulation by decreasing differentiation of effector T and B cells while promoting regulatory subsets. Vitamin D also modulates innate immune cells such as macrophages, monocytes and dendritic cells, and acts at the level of the blood-brain barrier reducing immune cell trafficking. Vitamin D exerts additional activity within the central nervous system reducing microglial and astrocytic activation. The immunomodulatory role of Vitamin D detected in animal models of multiple sclerosis has suggested its potential therapeutic use for treating multiple sclerosis. In this review, we focus on recent published data describing the biological effects of Vitamin D in animal models of multiple sclerosis on immune cells, blood-brain barrier function, activation of glial cells and its potential neuroprotective effects. Based on the current knowledge, we also discuss optimization of therapeutic interventions with Vitamin D in patients with multiple sclerosis, as well as new technologies allowing in-depth analysis of immune cell regulations by vitamin D.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Vitamin D deficiency has been associated with the risk of multiple sclerosis, disease activity and progression. Results from experiments, animal models and analysis of human samples from randomized controlled trials provide comprehensive data illustrating the pleiotropic actions of Vitamin D on the immune system. They globally result in immunomodulation by decreasing differentiation of effector T and B cells while promoting regulatory subsets. Vitamin D also modulates innate immune cells such as macrophages, monocytes and dendritic cells, and acts at the level of the blood-brain barrier reducing immune cell trafficking. Vitamin D exerts additional activity within the central nervous system reducing microglial and astrocytic activation. The immunomodulatory role of Vitamin D detected in animal models of multiple sclerosis has suggested its potential therapeutic use for treating multiple sclerosis. In this review, we focus on recent published data describing the biological effects of Vitamin D in animal models of multiple sclerosis on immune cells, blood-brain barrier function, activation of glial cells and its potential neuroprotective effects. Based on the current knowledge, we also discuss optimization of therapeutic interventions with Vitamin D in patients with multiple sclerosis, as well as new technologies allowing in-depth analysis of immune cell regulations by vitamin D. |
Yang, Cui; Blaize, Gaëtan; Marrocco, Rémi; Rouquié, Nelly; Bories, Cyrielle; Gador, Mylène; Mélique, Suzanne; Joulia, Emeline; Benamar, Mehdi; Dejean, Anne S.; Daniels-Treffandier, Hélène; Love, Paul E.; Fazilleau, Nicolas; Saoudi, Abdelhadi; Lesourne, Renaud THEMIS enhances the magnitude of normal and neuroinflammatory type 1 immune responses by promoting TCR-independent signals Article de journal Dans: Science Signaling, vol. 15, no. 742, p. eabl5343, 2022, (Publisher: American Association for the Advancement of Science). @article{yang_themis_2022,
title = {THEMIS enhances the magnitude of normal and neuroinflammatory type 1 immune responses by promoting TCR-independent signals},
author = {Yang, Cui and Blaize, Gaëtan and Marrocco, Rémi and Rouquié, Nelly and Bories, Cyrielle and Gador, Mylène and Mélique, Suzanne and Joulia, Emeline and Benamar, Mehdi and Dejean, Anne S. and Daniels-Treffandier, Hélène and Love, Paul E. and Fazilleau, Nicolas and Saoudi, Abdelhadi and Lesourne, Renaud},
url = {https://www.science.org/doi/abs/10.1126/scisignal.abl5343},
doi = {10.1126/scisignal.abl5343},
year = {2022},
date = {2022-01-01},
urldate = {2022-01-01},
journal = {Science Signaling},
volume = {15},
number = {742},
pages = {eabl5343},
note = {Publisher: American Association for the Advancement of Science},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2021
|
Tuzlak, Selma; Dejean, Anne S; Iannacone, Matteo; Quintana, Francisco J; Waisman, Ari; Ginhoux, Florent; Korn, Thomas; Becher, Burkhard Repositioning T cell polarization from single cytokines to complex help Article de journal Dans: Nat Immunol, vol. 22, no. 10, p. 1210–1217, 2021, ISSN: 1529-2916. @article{pmid34545250,
title = {Repositioning T cell polarization from single cytokines to complex help},
author = {Selma Tuzlak and Anne S Dejean and Matteo Iannacone and Francisco J Quintana and Ari Waisman and Florent Ginhoux and Thomas Korn and Burkhard Becher},
doi = {10.1038/s41590-021-01009-w},
issn = {1529-2916},
year = {2021},
date = {2021-10-01},
urldate = {2021-10-01},
journal = {Nat Immunol},
volume = {22},
number = {10},
pages = {1210--1217},
abstract = {When helper T (T) cell polarization was initially described three decades ago, the T cell universe grew dramatically. New subsets were described based on their expression of few specific cytokines. Beyond T1 and T2 cells, this led to the coining of various T17 and regulatory (T) cell subsets as well as T22, T25, follicular helper (T), T3, T5 and T9 cells. High-dimensional single-cell analysis revealed that a categorization of T cells into a single-cytokine-based nomenclature fails to capture the complexity and diversity of T cells. Similar to the simple nomenclature used to describe innate lymphoid cells (ILCs), we propose that T cell polarization should be categorized in terms of the help they provide to phagocytes (type 1), to B cells, eosinophils and mast cells (type 2) and to non-immune tissue cells, including the stroma and epithelium (type 3). Studying T cells based on their helper function and the cells they help, rather than phenotypic features such as individual analyzed cytokines or transcription factors, better captures T cell plasticity and conversion as well as the breadth of immune responses in vivo.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
When helper T (T) cell polarization was initially described three decades ago, the T cell universe grew dramatically. New subsets were described based on their expression of few specific cytokines. Beyond T1 and T2 cells, this led to the coining of various T17 and regulatory (T) cell subsets as well as T22, T25, follicular helper (T), T3, T5 and T9 cells. High-dimensional single-cell analysis revealed that a categorization of T cells into a single-cytokine-based nomenclature fails to capture the complexity and diversity of T cells. Similar to the simple nomenclature used to describe innate lymphoid cells (ILCs), we propose that T cell polarization should be categorized in terms of the help they provide to phagocytes (type 1), to B cells, eosinophils and mast cells (type 2) and to non-immune tissue cells, including the stroma and epithelium (type 3). Studying T cells based on their helper function and the cells they help, rather than phenotypic features such as individual analyzed cytokines or transcription factors, better captures T cell plasticity and conversion as well as the breadth of immune responses in vivo. |
Wiendl, Heinz; Gross, Catharina C; Bauer, Jan; Merkler, Doron; Prat, Alexandre; Liblau, Roland Fundamental mechanistic insights from rare but paradigmatic neuroimmunological diseases Article de journal Dans: Nat Rev Neurol, vol. 17, no. 7, p. 433–447, 2021, ISSN: 1759-4766. @article{pmid34050331,
title = {Fundamental mechanistic insights from rare but paradigmatic neuroimmunological diseases},
author = {Heinz Wiendl and Catharina C Gross and Jan Bauer and Doron Merkler and Alexandre Prat and Roland Liblau},
doi = {10.1038/s41582-021-00496-7},
issn = {1759-4766},
year = {2021},
date = {2021-07-01},
urldate = {2021-07-01},
journal = {Nat Rev Neurol},
volume = {17},
number = {7},
pages = {433--447},
abstract = {The pathophysiology of complex neuroimmunological diseases, such as multiple sclerosis and autoimmune encephalitis, remains puzzling - various mechanisms that are difficult to dissect seem to contribute, hampering the understanding of the processes involved. Some rare neuroimmunological diseases are easier to study because their presentation and pathogenesis are more homogeneous. The investigation of these diseases can provide fundamental insights into neuroimmunological pathomechanisms that can in turn be applied to more complex diseases. In this Review, we summarize key mechanistic insights into three such rare but paradigmatic neuroimmunological diseases - Susac syndrome, Rasmussen encephalitis and narcolepsy type 1 - and consider the implications of these insights for the study of other neuroimmunological diseases. In these diseases, the combination of findings in humans, different modalities of investigation and animal models has enabled the triangulation of evidence to validate and consolidate the pathomechanistic features and to develop diagnostic and therapeutic strategies; this approach has provided insights that are directly relevant to other neuroimmunological diseases and applicable in other contexts. We also outline how next-generation technologies and refined animal models can further improve our understanding of pathomechanisms, including cell-specific and antigen-specific CNS immune responses, thereby paving the way for the development of targeted therapeutic approaches.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The pathophysiology of complex neuroimmunological diseases, such as multiple sclerosis and autoimmune encephalitis, remains puzzling - various mechanisms that are difficult to dissect seem to contribute, hampering the understanding of the processes involved. Some rare neuroimmunological diseases are easier to study because their presentation and pathogenesis are more homogeneous. The investigation of these diseases can provide fundamental insights into neuroimmunological pathomechanisms that can in turn be applied to more complex diseases. In this Review, we summarize key mechanistic insights into three such rare but paradigmatic neuroimmunological diseases - Susac syndrome, Rasmussen encephalitis and narcolepsy type 1 - and consider the implications of these insights for the study of other neuroimmunological diseases. In these diseases, the combination of findings in humans, different modalities of investigation and animal models has enabled the triangulation of evidence to validate and consolidate the pathomechanistic features and to develop diagnostic and therapeutic strategies; this approach has provided insights that are directly relevant to other neuroimmunological diseases and applicable in other contexts. We also outline how next-generation technologies and refined animal models can further improve our understanding of pathomechanisms, including cell-specific and antigen-specific CNS immune responses, thereby paving the way for the development of targeted therapeutic approaches. |
Karmakar, Utsa; Chu, Julia Y; Sundaram, Kruthika; Astier, Anne L; Garside, Hannah; Hansen, Carsten G; Dransfield, Ian; Vermeren, Sonja Immune complex-induced apoptosis and concurrent immune complex clearance are anti-inflammatory neutrophil functions Article de journal Dans: Cell Death Dis, vol. 12, no. 4, p. 296, 2021, ISSN: 2041-4889. @article{pmid33741905,
title = {Immune complex-induced apoptosis and concurrent immune complex clearance are anti-inflammatory neutrophil functions},
author = {Utsa Karmakar and Julia Y Chu and Kruthika Sundaram and Anne L Astier and Hannah Garside and Carsten G Hansen and Ian Dransfield and Sonja Vermeren},
doi = {10.1038/s41419-021-03528-8},
issn = {2041-4889},
year = {2021},
date = {2021-03-01},
urldate = {2021-03-01},
journal = {Cell Death Dis},
volume = {12},
number = {4},
pages = {296},
abstract = {Persistent neutrophilic inflammation drives host damage in autoimmune diseases that are characterized by abundant immune complexes. Insoluble immune complexes (iICs) potently activate pro-inflammatory neutrophil effector functions. We and others have shown that iICs also promote resolution of inflammation via stimulation of neutrophil apoptosis. We demonstrate here that iICs trigger FcγRIIa-dependent neutrophil macropinocytosis, leading to the rapid uptake, and subsequent degradation of iICs. We provide evidence that concurrent iIC-induced neutrophil apoptosis is distinct from phagocytosis-induced cell death. First, uptake of iICs occurs by FcγRII-stimulated macropinocytosis, rather than phagocytosis. Second, production of reactive oxygen species, but not iIC-internalization is a pre-requisite for iIC-induced neutrophil apoptosis. Our findings identify a previously unknown mechanism by which neutrophils can remove pro-inflammatory iICs from the circulation. Together iIC clearance and iIC-induced neutrophil apoptosis may act to prevent the potential escalation of neutrophilic inflammation in response to iICs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Persistent neutrophilic inflammation drives host damage in autoimmune diseases that are characterized by abundant immune complexes. Insoluble immune complexes (iICs) potently activate pro-inflammatory neutrophil effector functions. We and others have shown that iICs also promote resolution of inflammation via stimulation of neutrophil apoptosis. We demonstrate here that iICs trigger FcγRIIa-dependent neutrophil macropinocytosis, leading to the rapid uptake, and subsequent degradation of iICs. We provide evidence that concurrent iIC-induced neutrophil apoptosis is distinct from phagocytosis-induced cell death. First, uptake of iICs occurs by FcγRII-stimulated macropinocytosis, rather than phagocytosis. Second, production of reactive oxygen species, but not iIC-internalization is a pre-requisite for iIC-induced neutrophil apoptosis. Our findings identify a previously unknown mechanism by which neutrophils can remove pro-inflammatory iICs from the circulation. Together iIC clearance and iIC-induced neutrophil apoptosis may act to prevent the potential escalation of neutrophilic inflammation in response to iICs. |
Rousset, S.; Lafaurie, M.; Guet-Revillet, H.; Protin, C.; Le Grusse, J.; Derumeaux, H.; Gandia, P.; Nourhashemi, F.; Sailler, L.; Sommet, A.; Delobel, P.; Martin-Blondel, G. Safety of Pyrazinamide for the Treatment of Tuberculosis in Older Patients Over 75 Years of Age: A Retrospective Monocentric Cohort Study Article de journal Dans: Drugs Aging, vol. 38, no. 1, p. 43-52, 2021, ISSN: 1179-1969 (Electronic)
1170-229X (Linking). @article{RN59,
title = {Safety of Pyrazinamide for the Treatment of Tuberculosis in Older Patients Over 75 Years of Age: A Retrospective Monocentric Cohort Study},
author = {Rousset, S. and Lafaurie, M. and Guet-Revillet, H. and Protin, C. and Le Grusse, J. and Derumeaux, H. and Gandia, P. and Nourhashemi, F. and Sailler, L. and Sommet, A. and Delobel, P. and Martin-Blondel, G.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/33145702},
doi = {10.1007/s40266-020-00811-9},
issn = {1179-1969 (Electronic)
1170-229X (Linking)},
year = {2021},
date = {2021-01-01},
journal = {Drugs Aging},
volume = {38},
number = {1},
pages = {43-52},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Guyonnet, S; Rolland, Y; Takeda, C; Ousset, P-J; Ader, I; Davezac, N; Dray, C; Fazilleau, N; Gourdy, P; Liblau, R; Parini, A; Payoux, P; Pénicaud, L; Rampon, C; Valet, P; Vergnolle, N; Andrieu, S; de Souto Barreto, P; Casteilla, L; Vellas, B The INSPIRE Bio-Resource Research Platform for Healthy Aging and Geroscience: Focus on the Human Translational Research Cohort (The INSPIRE-T Cohort) Article de journal Dans: J Frailty Aging, vol. 10, no. 2, p. 110–120, 2021, ISSN: 2260-1341. @article{pmid33575699,
title = {The INSPIRE Bio-Resource Research Platform for Healthy Aging and Geroscience: Focus on the Human Translational Research Cohort (The INSPIRE-T Cohort)},
author = {S Guyonnet and Y Rolland and C Takeda and P-J Ousset and I Ader and N Davezac and C Dray and N Fazilleau and P Gourdy and R Liblau and A Parini and P Payoux and L Pénicaud and C Rampon and P Valet and N Vergnolle and S Andrieu and P de Souto Barreto and L Casteilla and B Vellas},
doi = {10.14283/jfa.2020.38},
issn = {2260-1341},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {J Frailty Aging},
volume = {10},
number = {2},
pages = {110--120},
abstract = {BACKGROUND: The Geroscience field focuses on the core biological mechanisms of aging, which are involved in the onset of age-related diseases, as well as declines in intrinsic capacity (IC) (body functions) leading to dependency. A better understanding on how to measure the true age of an individual or biological aging is an essential step that may lead to the definition of putative markers capable of predicting healthy aging.nnOBJECTIVES: The main objective of the INStitute for Prevention healthy agIng and medicine Rejuvenative (INSPIRE) Platform initiative is to build a program for Geroscience and healthy aging research going from animal models to humans and the health care system. The specific aim of the INSPIRE human translational cohort (INSPIRE-T cohort) is to gather clinical, digital and imaging data, and perform relevant and extensive biobanking to allow basic and translational research on humans.nnMETHODS: The INSPIRE-T cohort consists in a population study comprising 1000 individuals in Toulouse and surrounding areas (France) of different ages (20 years or over - no upper limit for age) and functional capacity levels (from robustness to frailty, and even dependency) with follow-up over 10 years. Diversified data are collected annually in research facilities or at home according to standardized procedures. Between two annual visits, IC domains are monitored every 4-month by using the ICOPE Monitor app developed in collaboration with WHO. Once IC decline is confirmed, participants will have a clinical assessment and blood sampling to investigate markers of aging at the time IC declines are detected. Biospecimens include blood, urine, saliva, and dental plaque that are collected from all subjects at baseline and then, annually. Nasopharyngeal swabs and cutaneous surface samples are collected in a large subgroup of subjects every two years. Feces, hair bulb and skin biopsy are collected optionally at the baseline visit and will be performed again during the longitudinal follow up.nnEXPECTED RESULTS: Recruitment started on October 2019 and is expected to last for two years. Bio-resources collected and explored in the INSPIRE-T cohort will be available for academic and industry partners aiming to identify robust (set of) markers of aging, age-related diseases and IC evolution that could be pharmacologically or non-pharmacologically targetable. The INSPIRE-T will also aim to develop an integrative approach to explore the use of innovative technologies and a new, function and person-centered health care pathway that will promote a healthy aging.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
BACKGROUND: The Geroscience field focuses on the core biological mechanisms of aging, which are involved in the onset of age-related diseases, as well as declines in intrinsic capacity (IC) (body functions) leading to dependency. A better understanding on how to measure the true age of an individual or biological aging is an essential step that may lead to the definition of putative markers capable of predicting healthy aging.nnOBJECTIVES: The main objective of the INStitute for Prevention healthy agIng and medicine Rejuvenative (INSPIRE) Platform initiative is to build a program for Geroscience and healthy aging research going from animal models to humans and the health care system. The specific aim of the INSPIRE human translational cohort (INSPIRE-T cohort) is to gather clinical, digital and imaging data, and perform relevant and extensive biobanking to allow basic and translational research on humans.nnMETHODS: The INSPIRE-T cohort consists in a population study comprising 1000 individuals in Toulouse and surrounding areas (France) of different ages (20 years or over - no upper limit for age) and functional capacity levels (from robustness to frailty, and even dependency) with follow-up over 10 years. Diversified data are collected annually in research facilities or at home according to standardized procedures. Between two annual visits, IC domains are monitored every 4-month by using the ICOPE Monitor app developed in collaboration with WHO. Once IC decline is confirmed, participants will have a clinical assessment and blood sampling to investigate markers of aging at the time IC declines are detected. Biospecimens include blood, urine, saliva, and dental plaque that are collected from all subjects at baseline and then, annually. Nasopharyngeal swabs and cutaneous surface samples are collected in a large subgroup of subjects every two years. Feces, hair bulb and skin biopsy are collected optionally at the baseline visit and will be performed again during the longitudinal follow up.nnEXPECTED RESULTS: Recruitment started on October 2019 and is expected to last for two years. Bio-resources collected and explored in the INSPIRE-T cohort will be available for academic and industry partners aiming to identify robust (set of) markers of aging, age-related diseases and IC evolution that could be pharmacologically or non-pharmacologically targetable. The INSPIRE-T will also aim to develop an integrative approach to explore the use of innovative technologies and a new, function and person-centered health care pathway that will promote a healthy aging. |
de Souto Barreto, P; Guyonnet, S; Ader, I; Andrieu, S; Casteilla, L; Davezac, N; Dray, C; Fazilleau, N; Gourdy, P; Liblau, R; Parini, A; Payoux, P; Pénicaud, L; Rampon, C; Rolland, Y; Valet, P; Vergnolle, N; Vellas, B The INSPIRE Research Initiative: A Program for GeroScience and Healthy Aging Research Going from Animal Models to Humans and the Healthcare System Article de journal Dans: J Frailty Aging, vol. 10, no. 2, p. 86–93, 2021, ISSN: 2260-1341. @article{pmid33575696,
title = {The INSPIRE Research Initiative: A Program for GeroScience and Healthy Aging Research Going from Animal Models to Humans and the Healthcare System},
author = {P de Souto Barreto and S Guyonnet and I Ader and S Andrieu and L Casteilla and N Davezac and C Dray and N Fazilleau and P Gourdy and R Liblau and A Parini and P Payoux and L Pénicaud and C Rampon and Y Rolland and P Valet and N Vergnolle and B Vellas},
doi = {10.14283/jfa.2020.18},
issn = {2260-1341},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {J Frailty Aging},
volume = {10},
number = {2},
pages = {86--93},
abstract = {Aging is the most important risk factor for the onset of several chronic diseases and functional decline. Understanding the interplays between biological aging and the biology of diseases and functional loss as well as integrating a function-centered approach to the care pathway of older adults are crucial steps towards the elaboration of preventive strategies (both pharmacological and non-pharmacological) against the onset and severity of burdensome chronic conditions during aging. In order to tackle these two crucial challenges, ie, how both the manipulation of biological aging and the implementation of a function-centered care pathway (the Integrated Care for Older People (ICOPE) model of the World Health Organization) may contribute to the trajectories of healthy aging, a new initiative on Gerosciences was built: the INSPIRE research program. The present article describes the scientific background on which the foundations of the INSPIRE program have been constructed and provides the general lines of this initiative that involves researchers from basic and translational science, clinical gerontology, geriatrics and primary care, and public health.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Aging is the most important risk factor for the onset of several chronic diseases and functional decline. Understanding the interplays between biological aging and the biology of diseases and functional loss as well as integrating a function-centered approach to the care pathway of older adults are crucial steps towards the elaboration of preventive strategies (both pharmacological and non-pharmacological) against the onset and severity of burdensome chronic conditions during aging. In order to tackle these two crucial challenges, ie, how both the manipulation of biological aging and the implementation of a function-centered care pathway (the Integrated Care for Older People (ICOPE) model of the World Health Organization) may contribute to the trajectories of healthy aging, a new initiative on Gerosciences was built: the INSPIRE research program. The present article describes the scientific background on which the foundations of the INSPIRE program have been constructed and provides the general lines of this initiative that involves researchers from basic and translational science, clinical gerontology, geriatrics and primary care, and public health. |
2020
|
Yshii, L.; Bost, C.; Liblau, R. Immunological Bases of Paraneoplastic Cerebellar Degeneration and Therapeutic Implications Article de journal Dans: Front Immunol, vol. 11, p. 991, 2020, ISSN: 1664-3224 (Electronic)
1664-3224 (Linking). @article{RN55,
title = {Immunological Bases of Paraneoplastic Cerebellar Degeneration and Therapeutic Implications},
author = {Yshii, L. and Bost, C. and Liblau, R.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/32655545},
doi = {10.3389/fimmu.2020.00991},
issn = {1664-3224 (Electronic)
1664-3224 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Front Immunol},
volume = {11},
pages = {991},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pavlovic, M.; Gross, C.; Chili, C.; Secher, T.; Treiner, E. MAIT Cells Display a Specific Response to Type 1 IFN Underlying the Adjuvant Effect of TLR7/8 Ligands Article de journal Dans: Front Immunol, vol. 11, p. 2097, 2020, ISSN: 1664-3224 (Electronic)
1664-3224 (Linking). @article{RN57,
title = {MAIT Cells Display a Specific Response to Type 1 IFN Underlying the Adjuvant Effect of TLR7/8 Ligands},
author = {Pavlovic, M. and Gross, C. and Chili, C. and Secher, T. and Treiner, E.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/33013883},
doi = {10.3389/fimmu.2020.02097},
issn = {1664-3224 (Electronic)
1664-3224 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Front Immunol},
volume = {11},
pages = {2097},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Malviya, M.; Saoudi, A.; Bauer, J.; Fillatreau, S.; Liblau, R. Treatment of experimental autoimmune encephalomyelitis with engineered bi-specific Foxp3+ regulatory CD4+ T cells Article de journal Dans: J Autoimmun, vol. 108, p. 102401, 2020, ISSN: 1095-9157 (Electronic) 0896-8411 (Linking). @article{RN54,
title = {Treatment of experimental autoimmune encephalomyelitis with engineered bi-specific Foxp3+ regulatory CD4+ T cells},
author = {Malviya, M. and Saoudi, A. and Bauer, J. and Fillatreau, S. and Liblau, R.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31948790},
doi = {10.1016/j.jaut.2020.102401},
issn = {1095-9157 (Electronic) 0896-8411 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {J Autoimmun},
volume = {108},
pages = {102401},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Killick, J.; Hay, J.; Morandi, E.; Vermeren, S.; Kari, S.; Angles, T.; Williams, A.; Damoiseaux, J.; Astier, A. L. Vitamin D/CD46 Crosstalk in Human T Cells in Multiple Sclerosis Article de journal Dans: Front Immunol, vol. 11, p. 598727, 2020, ISSN: 1664-3224 (Electronic)
1664-3224 (Linking). @article{RN56,
title = {Vitamin D/CD46 Crosstalk in Human T Cells in Multiple Sclerosis},
author = {Killick, J. and Hay, J. and Morandi, E. and Vermeren, S. and Kari, S. and Angles, T. and Williams, A. and Damoiseaux, J. and Astier, A. L.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/33329593},
doi = {10.3389/fimmu.2020.598727},
issn = {1664-3224 (Electronic)
1664-3224 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Front Immunol},
volume = {11},
pages = {598727},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Kamar, N.; Esposito, L.; Hebral, A. L.; Guitard, J.; Del Bello, A. Specific organization for in-hospital belatacept infusion to avoid nosocomial transmission during the SARS-CoV-2 pandemic Article de journal Dans: Am J Transplant, vol. 20, no. 10, p. 2962-2963, 2020, ISSN: 1600-6143 (Electronic)
1600-6135 (Linking). @article{RN61,
title = {Specific organization for in-hospital belatacept infusion to avoid nosocomial transmission during the SARS-CoV-2 pandemic},
author = {Kamar, N. and Esposito, L. and Hebral, A. L. and Guitard, J. and Del Bello, A.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/32449188},
doi = {10.1111/ajt.16074},
issn = {1600-6143 (Electronic)
1600-6135 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Am J Transplant},
volume = {20},
number = {10},
pages = {2962-2963},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Del Bello, A.; Kamar, N.; Treiner, E. T cell reconstitution after lymphocyte depletion features a different pattern of inhibitory receptor expression in ABO- versus HLA-incompatible kidney transplant recipients Article de journal Dans: Clin Exp Immunol, vol. 200, no. 1, p. 89-104, 2020, ISSN: 1365-2249 (Electronic)
0009-9104 (Linking). @article{RN58,
title = {T cell reconstitution after lymphocyte depletion features a different pattern of inhibitory receptor expression in ABO- versus HLA-incompatible kidney transplant recipients},
author = {Del Bello, A. and Kamar, N. and Treiner, E.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31869432},
doi = {10.1111/cei.13412},
issn = {1365-2249 (Electronic)
0009-9104 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Clin Exp Immunol},
volume = {200},
number = {1},
pages = {89-104},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Cohen, M.; Mondot, L.; Bucciarelli, F.; Pignolet, B.; Laplaud, D. A.; Wiertlewski, S.; Brochet, B.; Ruet, A.; Defer, G.; Derache, N.; Vermersch, P.; Zephir, H.; Debouverie, M.; Mathey, G.; Berger, E.; Cappe, C.; Labauge, P.; Carra, C.; De Seze, J.; Bigaut, K.; Brassat, D.; Lebrun-Frenay, C. BEST-MS: A prospective head-to-head comparative study of natalizumab and fingolimod in active relapsing MS Article de journal Dans: Mult Scler, p. 1352458520969145, 2020, ISSN: 1477-0970 (Electronic)
1352-4585 (Linking). @article{RN60,
title = {BEST-MS: A prospective head-to-head comparative study of natalizumab and fingolimod in active relapsing MS},
author = {Cohen, M. and Mondot, L. and Bucciarelli, F. and Pignolet, B. and Laplaud, D. A. and Wiertlewski, S. and Brochet, B. and Ruet, A. and Defer, G. and Derache, N. and Vermersch, P. and Zephir, H. and Debouverie, M. and Mathey, G. and Berger, E. and Cappe, C. and Labauge, P. and Carra, C. and De Seze, J. and Bigaut, K. and Brassat, D. and Lebrun-Frenay, C.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/33124504},
doi = {10.1177/1352458520969145},
issn = {1477-0970 (Electronic)
1352-4585 (Linking)},
year = {2020},
date = {2020-01-01},
journal = {Mult Scler},
pages = {1352458520969145},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2019
|
Eyoh, Enwono; McCallum, Patrick; Killick, Justin; Amanfo, Seth; Mutapi, Francisca; Astier, Anne L The anthelmintic drug praziquantel promotes human Tr1 differentiation Article de journal Dans: Immunol Cell Biol, vol. 97, no. 5, p. 512–518, 2019, ISSN: 1440-1711. @article{pmid30623486,
title = {The anthelmintic drug praziquantel promotes human Tr1 differentiation},
author = {Enwono Eyoh and Patrick McCallum and Justin Killick and Seth Amanfo and Francisca Mutapi and Anne L Astier},
doi = {10.1111/imcb.12229},
issn = {1440-1711},
year = {2019},
date = {2019-05-01},
urldate = {2019-05-01},
journal = {Immunol Cell Biol},
volume = {97},
number = {5},
pages = {512--518},
abstract = {Praziquantel (PZQ) is an anthelminthic human and veterinary drug used to treat trematode and cestode worms. Changes in immune responses have been demonstrated in humans following curative PZQ treatment of schistosome infections. These changes have been attributed to the removal of immunosupressive worms and immune responses to parasite antigens exposed from dying worms. To date, there has been no study investigating the potential direct effect of PZQ on the host immune cells. Herein, we analyzed the effect of PZQ on human CD4 T cells classically costimulated by CD3/CD28 or costimulated by the complement regulator CD46 to induce Type 1 regulatory T cells (Tr1). Our results show that PZQ enhanced T-cell proliferation, increased secretion of IL-17 and IL-10 but had no effect on secretion of GM-CSF or IFNγ. Moreover, PZQ increased the coexpression of CD49b and LAG-3, a hallmark of Tr1 cells, suggesting increased Tr1 differentiation. Indeed, supernatants from PZQ-treated cells were able to decrease bystander T-cell activation, and this was partly reduced when blocking IL-10. Hence, our study demonstrates that PZQ directly modulates human T-cell activation and promotes Tr1 differentiation, suggesting that PZQ may have immunomodulatory functions in parasite-unrelated human inflammatory diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Praziquantel (PZQ) is an anthelminthic human and veterinary drug used to treat trematode and cestode worms. Changes in immune responses have been demonstrated in humans following curative PZQ treatment of schistosome infections. These changes have been attributed to the removal of immunosupressive worms and immune responses to parasite antigens exposed from dying worms. To date, there has been no study investigating the potential direct effect of PZQ on the host immune cells. Herein, we analyzed the effect of PZQ on human CD4 T cells classically costimulated by CD3/CD28 or costimulated by the complement regulator CD46 to induce Type 1 regulatory T cells (Tr1). Our results show that PZQ enhanced T-cell proliferation, increased secretion of IL-17 and IL-10 but had no effect on secretion of GM-CSF or IFNγ. Moreover, PZQ increased the coexpression of CD49b and LAG-3, a hallmark of Tr1 cells, suggesting increased Tr1 differentiation. Indeed, supernatants from PZQ-treated cells were able to decrease bystander T-cell activation, and this was partly reduced when blocking IL-10. Hence, our study demonstrates that PZQ directly modulates human T-cell activation and promotes Tr1 differentiation, suggesting that PZQ may have immunomodulatory functions in parasite-unrelated human inflammatory diseases. |
Rousset, S.; Treiner, E.; Moulis, G.; Pugnet, G.; Astudillo, L.; Paricaud, K.; Puissant-Lubrano, B.; Arlet, P.; Blancher, A.; Sailler, L. High rate of indeterminate results of the QuantiFERON-TB Gold in-tube test, third generation, in patients with systemic vasculitis Article de journal Dans: Rheumatology (Oxford), 2019, ISSN: 1462-0332 (Electronic)
1462-0324 (Linking). @article{RN47b,
title = {High rate of indeterminate results of the QuantiFERON-TB Gold in-tube test, third generation, in patients with systemic vasculitis},
author = {Rousset, S. and Treiner, E. and Moulis, G. and Pugnet, G. and Astudillo, L. and Paricaud, K. and Puissant-Lubrano, B. and Arlet, P. and Blancher, A. and Sailler, L.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31518431},
doi = {10.1093/rheumatology/kez390},
issn = {1462-0332 (Electronic)
1462-0324 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Rheumatology (Oxford)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Aron Badin, R.; Bugi, A.; Williams, S.; Vadori, M.; Michael, M.; Jan, C.; Nassi, A.; Lecourtois, S.; Blancher, A.; Cozzi, E.; Hantraye, P.; Perrier, A. L. MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates Article de journal Dans: Nat Commun, vol. 10, no. 1, p. 4357, 2019, ISSN: 2041-1723 (Electronic)
2041-1723 (Linking). @article{RN45b,
title = {MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates},
author = {Aron Badin, R. and Bugi, A. and Williams, S. and Vadori, M. and Michael, M. and Jan, C. and Nassi, A. and Lecourtois, S. and Blancher, A. and Cozzi, E. and Hantraye, P. and Perrier, A. L.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31554807},
doi = {10.1038/s41467-019-12324-0},
issn = {2041-1723 (Electronic)
2041-1723 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Nat Commun},
volume = {10},
number = {1},
pages = {4357},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bassetti, C. L. A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B. R.; Lammers, G. J.; Liblau, R. S.; Luppi, P. H.; Mayer, G.; Pollmacher, T.; Sakurai, T.; Sallusto, F.; Scammell, T. E.; Tafti, M.; Dauvilliers, Y. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment Article de journal Dans: Nat Rev Neurol, vol. 15, no. 9, p. 519-539, 2019, ISSN: 1759-4766 (Electronic)
1759-4758 (Linking). @article{RN41b,
title = {Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment},
author = {Bassetti, C. L. A. and Adamantidis, A. and Burdakov, D. and Han, F. and Gay, S. and Kallweit, U. and Khatami, R. and Koning, F. and Kornum, B. R. and Lammers, G. J. and Liblau, R. S. and Luppi, P. H. and Mayer, G. and Pollmacher, T. and Sakurai, T. and Sallusto, F. and Scammell, T. E. and Tafti, M. and Dauvilliers, Y.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31324898},
doi = {10.1038/s41582-019-0226-9},
issn = {1759-4766 (Electronic)
1759-4758 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Nat Rev Neurol},
volume = {15},
number = {9},
pages = {519-539},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Beltran, E.; Nguyen, X. H.; Queriault, C.; Barateau, L.; Dauvilliers, Y.; Dornmair, K.; Liblau, R. S. Shared T cell receptor chains in blood memory CD4(+) T cells of narcolepsy type 1 patients Article de journal Dans: J Autoimmun, vol. 100, p. 1-6, 2019, ISSN: 1095-9157 (Electronic)
0896-8411 (Linking). @article{RN42,
title = {Shared T cell receptor chains in blood memory CD4(+) T cells of narcolepsy type 1 patients},
author = {Beltran, E. and Nguyen, X. H. and Queriault, C. and Barateau, L. and Dauvilliers, Y. and Dornmair, K. and Liblau, R. S.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30948158},
doi = {10.1016/j.jaut.2019.03.010},
issn = {1095-9157 (Electronic)
0896-8411 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {J Autoimmun},
volume = {100},
pages = {1-6},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Dejean, A. S.; Joulia, E.; Walzer, T. The role of Eomes in human CD4 T cell differentiation: A question of context Article de journal Dans: Eur J Immunol, vol. 49, no. 1, p. 38-41, 2019, ISSN: 1521-4141 (Electronic)
0014-2980 (Linking). @article{RN48b,
title = {The role of Eomes in human CD4 T cell differentiation: A question of context},
author = {Dejean, A. S. and Joulia, E. and Walzer, T.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30536524},
doi = {10.1002/eji.201848000},
issn = {1521-4141 (Electronic)
0014-2980 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Eur J Immunol},
volume = {49},
number = {1},
pages = {38-41},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yshii, L.; Pignolet, B.; Maure, E.; Pierau, M.; Brunner-Weinzierl, M.; Hartley, O.; Bauer, J.; Liblau, R. IFN-gamma is a therapeutic target in paraneoplastic cerebellar degeneration Article de journal Dans: JCI Insight, vol. 4, no. 7, 2019, ISSN: 2379-3708 (Electronic)
2379-3708 (Linking). @article{RN32b,
title = {IFN-gamma is a therapeutic target in paraneoplastic cerebellar degeneration},
author = {Yshii, L. and Pignolet, B. and Maure, E. and Pierau, M. and Brunner-Weinzierl, M. and Hartley, O. and Bauer, J. and Liblau, R.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30944244},
doi = {10.1172/jci.insight.127001},
issn = {2379-3708 (Electronic)
2379-3708 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {JCI Insight},
volume = {4},
number = {7},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Giner-Delgado, C.; Villatoro, S.; Lerga-Jaso, J.; Gaya-Vidal, M.; Oliva, M.; Castellano, D.; Pantano, L.; Bitarello, B. D.; Izquierdo, D.; Noguera, I.; Olalde, I.; Delprat, A.; Blancher, A.; Lalueza-Fox, C.; Esko, T.; O'Reilly, P. F.; Andres, A. M.; Ferretti, L.; Puig, M.; Caceres, M. Evolutionary and functional impact of common polymorphic inversions in the human genome Article de journal Dans: Nat Commun, vol. 10, no. 1, p. 4222, 2019, ISSN: 2041-1723 (Electronic)
2041-1723 (Linking). @article{RN46b,
title = {Evolutionary and functional impact of common polymorphic inversions in the human genome},
author = {Giner-Delgado, C. and Villatoro, S. and Lerga-Jaso, J. and Gaya-Vidal, M. and Oliva, M. and Castellano, D. and Pantano, L. and Bitarello, B. D. and Izquierdo, D. and Noguera, I. and Olalde, I. and Delprat, A. and Blancher, A. and Lalueza-Fox, C. and Esko, T. and O'Reilly, P. F. and Andres, A. M. and Ferretti, L. and Puig, M. and Caceres, M.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31530810},
doi = {10.1038/s41467-019-12173-x},
issn = {2041-1723 (Electronic)
2041-1723 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Nat Commun},
volume = {10},
number = {1},
pages = {4222},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Walter, O.; Treiner, E.; Bonneville, F.; Mengelle, C.; Vergez, F.; Lerebours, F.; Delobel, P.; Liblau, R.; Martin-Blondel, G.; Immune Checkpoint Inhibitors in, P. M. L. Study Group Treatment of Progressive Multifocal Leukoencephalopathy with Nivolumab Article de journal Dans: N Engl J Med, vol. 380, no. 17, p. 1674-1676, 2019, ISSN: 1533-4406 (Electronic)
0028-4793 (Linking). @article{RN27b,
title = {Treatment of Progressive Multifocal Leukoencephalopathy with Nivolumab},
author = {Walter, O. and Treiner, E. and Bonneville, F. and Mengelle, C. and Vergez, F. and Lerebours, F. and Delobel, P. and Liblau, R. and Martin-Blondel, G. and Immune Checkpoint Inhibitors in, P. M. L. Study Group},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30969500},
doi = {10.1056/NEJMc1816198},
issn = {1533-4406 (Electronic)
0028-4793 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {N Engl J Med},
volume = {380},
number = {17},
pages = {1674-1676},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Klotz, L.; Eschborn, M.; Lindner, M.; Liebmann, M.; Herold, M.; Janoschka, C.; Torres Garrido, B.; Schulte-Mecklenbeck, A.; Gross, C. C.; Breuer, J.; Hundehege, P.; Posevitz, V.; Pignolet, B.; Nebel, G.; Glander, S.; Freise, N.; Austermann, J.; Wirth, T.; Campbell, G. R.; Schneider-Hohendorf, T.; Eveslage, M.; Brassat, D.; Schwab, N.; Loser, K.; Roth, J.; Busch, K. B.; Stoll, M.; Mahad, D. J.; Meuth, S. G.; Turner, T.; Bar-Or, A.; Wiendl, H. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects Article de journal Dans: Sci Transl Med, vol. 11, no. 490, 2019, ISSN: 1946-6242 (Electronic)
1946-6234 (Linking). @article{RN43b,
title = {Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects},
author = {Klotz, L. and Eschborn, M. and Lindner, M. and Liebmann, M. and Herold, M. and Janoschka, C. and Torres Garrido, B. and Schulte-Mecklenbeck, A. and Gross, C. C. and Breuer, J. and Hundehege, P. and Posevitz, V. and Pignolet, B. and Nebel, G. and Glander, S. and Freise, N. and Austermann, J. and Wirth, T. and Campbell, G. R. and Schneider-Hohendorf, T. and Eveslage, M. and Brassat, D. and Schwab, N. and Loser, K. and Roth, J. and Busch, K. B. and Stoll, M. and Mahad, D. J. and Meuth, S. G. and Turner, T. and Bar-Or, A. and Wiendl, H.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31043571},
doi = {10.1126/scitranslmed.aao5563},
issn = {1946-6242 (Electronic)
1946-6234 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Sci Transl Med},
volume = {11},
number = {490},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Schwab, N.; Schneider-Hohendorf, T.; Pignolet, B.; Bucciarelli, F.; Scandella, L.; Ciron, J.; Biotti, D.; Lebrun-Frenay, C.; Mathey, G.; Clavelou, P.; Pelletier, J.; Ostkamp, P.; Meinl, I.; Windhagen, S.; Klotz, L.; Gross, C. C.; Meuth, S. G.; Deisenhammer, F.; Brassat, D.; Wiendl, H.; Best, M. S. Study Group Prospective validation of the PML risk biomarker l-selectin and influence of natalizumab extended intervals Article de journal Dans: Neurology, vol. 93, no. 12, p. 550-554, 2019, ISSN: 1526-632X (Electronic)
0028-3878 (Linking). @article{RN44b,
title = {Prospective validation of the PML risk biomarker l-selectin and influence of natalizumab extended intervals},
author = {Schwab, N. and Schneider-Hohendorf, T. and Pignolet, B. and Bucciarelli, F. and Scandella, L. and Ciron, J. and Biotti, D. and Lebrun-Frenay, C. and Mathey, G. and Clavelou, P. and Pelletier, J. and Ostkamp, P. and Meinl, I. and Windhagen, S. and Klotz, L. and Gross, C. C. and Meuth, S. G. and Deisenhammer, F. and Brassat, D. and Wiendl, H. and Best, M. S. Study Group},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31439633},
doi = {10.1212/WNL.0000000000008135},
issn = {1526-632X (Electronic)
0028-3878 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Neurology},
volume = {93},
number = {12},
pages = {550-554},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Salvioni, A.; Belloy, M.; Lebourg, A.; Bassot, E.; Cantaloube-Ferrieu, V.; Vasseur, V.; Blanie, S.; Liblau, R. S.; Suberbielle, E.; Robey, E. A.; Blanchard, N. Robust Control of a Brain-Persisting Parasite through MHC I Presentation by Infected Neurons Article de journal Dans: Cell Rep, vol. 27, no. 11, p. 3254-3268 e8, 2019, ISSN: 2211-1247 (Electronic). @article{RN40b,
title = {Robust Control of a Brain-Persisting Parasite through MHC I Presentation by Infected Neurons},
author = {Salvioni, A. and Belloy, M. and Lebourg, A. and Bassot, E. and Cantaloube-Ferrieu, V. and Vasseur, V. and Blanie, S. and Liblau, R. S. and Suberbielle, E. and Robey, E. A. and Blanchard, N.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31189109},
doi = {10.1016/j.celrep.2019.05.051},
issn = {2211-1247 (Electronic)},
year = {2019},
date = {2019-01-01},
journal = {Cell Rep},
volume = {27},
number = {11},
pages = {3254-3268 e8},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2018
|
Melliez, H.; Mary-Krause, M.; Bocket, L.; Guiguet, M.; Abgrall, S.; De Truchis, P.; Katlama, C.; Martin-Blondel, G.; Henn, A.; Revest, M.; Robineau, O.; Khuong-Josses, M. A.; Canestri, A.; De Castro, N.; Joly, V.; Mokhtari, S.; Risso, K.; Gasnault, J.; Costagliola, D.; French Hospital Database on, H. I. V. Risk of Progressive Multifocal Leukoencephalopathy in the Combination Antiretroviral Therapy Era in the French Hospital Database on Human Immunodeficiency Virus (ANRS-C4) Article de journal Dans: Clin Infect Dis, vol. 67, no. 2, p. 275-282, 2018, ISSN: 1537-6591 (Electronic)
1058-4838 (Linking). @article{RN51b,
title = {Risk of Progressive Multifocal Leukoencephalopathy in the Combination Antiretroviral Therapy Era in the French Hospital Database on Human Immunodeficiency Virus (ANRS-C4)},
author = {Melliez, H. and Mary-Krause, M. and Bocket, L. and Guiguet, M. and Abgrall, S. and De Truchis, P. and Katlama, C. and Martin-Blondel, G. and Henn, A. and Revest, M. and Robineau, O. and Khuong-Josses, M. A. and Canestri, A. and De Castro, N. and Joly, V. and Mokhtari, S. and Risso, K. and Gasnault, J. and Costagliola, D. and French Hospital Database on, H. I. V.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29635465},
doi = {10.1093/cid/ciy074},
issn = {1537-6591 (Electronic)
1058-4838 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Clin Infect Dis},
volume = {67},
number = {2},
pages = {275-282},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Eissmann, M. F.; Dijkstra, C.; Wouters, M. A.; Baloyan, D.; Mouradov, D.; Nguyen, P. M.; Davalos-Salas, M.; Putoczki, T. L.; Sieber, O. M.; Mariadason, J. M.; Ernst, M.; Masson, F. Interleukin 33 Signaling Restrains Sporadic Colon Cancer in an Interferon-gamma-Dependent Manner Article de journal Dans: Cancer Immunol Res, vol. 6, no. 4, p. 409-421, 2018, ISSN: 2326-6074 (Electronic) 2326-6066 (Linking). @article{RN1b,
title = {Interleukin 33 Signaling Restrains Sporadic Colon Cancer in an Interferon-gamma-Dependent Manner},
author = {Eissmann, M. F. and Dijkstra, C. and Wouters, M. A. and Baloyan, D. and Mouradov, D. and Nguyen, P. M. and Davalos-Salas, M. and Putoczki, T. L. and Sieber, O. M. and Mariadason, J. M. and Ernst, M. and Masson, F.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29463593},
doi = {10.1158/2326-6066.CIR-17-0218},
issn = {2326-6074 (Electronic) 2326-6066 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Cancer Immunol Res},
volume = {6},
number = {4},
pages = {409-421},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Januel, E.; Martin-Blondel, G.; Lamirel, C.; Picard, H.; Pialoux, G.; Katlama, C.; Cochereau, I.; Lescure, F. X.; Moulignier, A. Do CCR5 antagonists improve the overall survival of patients with AIDS-related progressive multifocal leucoencephalopathy? Article de journal Dans: J Neurol Neurosurg Psychiatry, vol. 89, no. 10, p. 1125-1126, 2018, ISSN: 1468-330X (Electronic)
0022-3050 (Linking). @article{RN53b,
title = {Do CCR5 antagonists improve the overall survival of patients with AIDS-related progressive multifocal leucoencephalopathy?},
author = {Januel, E. and Martin-Blondel, G. and Lamirel, C. and Picard, H. and Pialoux, G. and Katlama, C. and Cochereau, I. and Lescure, F. X. and Moulignier, A.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29305436},
doi = {10.1136/jnnp-2017-317203},
issn = {1468-330X (Electronic)
0022-3050 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {J Neurol Neurosurg Psychiatry},
volume = {89},
number = {10},
pages = {1125-1126},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Mansuy, J. M.; Lhomme, S.; Cazabat, M.; Pasquier, C.; Martin-Blondel, G.; Izopet, J. Detection of Zika, dengue and chikungunya viruses using single-reaction multiplex real-time RT-PCR Article de journal Dans: Diagn Microbiol Infect Dis, vol. 92, no. 4, p. 284-287, 2018, ISSN: 1879-0070 (Electronic)
0732-8893 (Linking). @article{RN52b,
title = {Detection of Zika, dengue and chikungunya viruses using single-reaction multiplex real-time RT-PCR},
author = {Mansuy, J. M. and Lhomme, S. and Cazabat, M. and Pasquier, C. and Martin-Blondel, G. and Izopet, J.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30029808},
doi = {10.1016/j.diagmicrobio.2018.06.019},
issn = {1879-0070 (Electronic)
0732-8893 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Diagn Microbiol Infect Dis},
volume = {92},
number = {4},
pages = {284-287},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Montalban, X.; Gold, R.; Thompson, A. J.; Otero-Romero, S.; Amato, M. P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; Hartung, H. P.; Havrdova, E.; Hemmer, B.; Kappos, L.; Liblau, R.; Lubetzki, C.; Marcus, E.; Miller, D. H.; Olsson, T.; Pilling, S.; Selmaj, K.; Siva, A.; Sorensen, P. S.; Sormani, M. P.; Thalheim, C.; Wiendl, H.; Zipp, F. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis Article de journal Dans: Mult Scler, vol. 24, no. 2, p. 96-120, 2018, ISSN: 1477-0970 (Electronic)
1352-4585 (Linking). @article{RN49b,
title = {ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis},
author = {Montalban, X. and Gold, R. and Thompson, A. J. and Otero-Romero, S. and Amato, M. P. and Chandraratna, D. and Clanet, M. and Comi, G. and Derfuss, T. and Fazekas, F. and Hartung, H. P. and Havrdova, E. and Hemmer, B. and Kappos, L. and Liblau, R. and Lubetzki, C. and Marcus, E. and Miller, D. H. and Olsson, T. and Pilling, S. and Selmaj, K. and Siva, A. and Sorensen, P. S. and Sormani, M. P. and Thalheim, C. and Wiendl, H. and Zipp, F.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29353550},
doi = {10.1177/1352458517751049},
issn = {1477-0970 (Electronic)
1352-4585 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Mult Scler},
volume = {24},
number = {2},
pages = {96-120},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Tugues, S.; Amorim, A.; Spath, S.; Martin-Blondel, G.; Schreiner, B.; De Feo, D.; Lutz, M.; Guscetti, F.; Apostolova, P.; Haftmann, C.; Hasselblatt, P.; Nunez, N. G.; Hottiger, M. O.; van den Broek, M.; Manz, M. G.; Zeiser, R.; Becher, B. Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells Article de journal Dans: Sci Transl Med, vol. 10, no. 469, 2018, ISSN: 1946-6242 (Electronic)
1946-6234 (Linking). @article{RN50b,
title = {Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells},
author = {Tugues, S. and Amorim, A. and Spath, S. and Martin-Blondel, G. and Schreiner, B. and De Feo, D. and Lutz, M. and Guscetti, F. and Apostolova, P. and Haftmann, C. and Hasselblatt, P. and Nunez, N. G. and Hottiger, M. O. and van den Broek, M. and Manz, M. G. and Zeiser, R. and Becher, B.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30487251},
doi = {10.1126/scitranslmed.aat8410},
issn = {1946-6242 (Electronic)
1946-6234 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Sci Transl Med},
volume = {10},
number = {469},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Liblau, R. S. Put to sleep by immune cells Article de journal Dans: Nature, vol. 562, no. 7725, p. 46-48, 2018, ISSN: 1476-4687 (Electronic)
0028-0836 (Linking). @article{RN22b,
title = {Put to sleep by immune cells},
author = {Liblau, R. S.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30275550},
doi = {10.1038/d41586-018-06666-w},
issn = {1476-4687 (Electronic)
0028-0836 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Nature},
volume = {562},
number = {7725},
pages = {46-48},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Gaud, G.; Roncagalli, R.; Chaoui, K.; Bernard, I.; Familiades, J.; Colacios, C.; Kassem, S.; Monsarrat, B.; Burlet-Schiltz, O.; de Peredo, A. G.; Malissen, B.; Saoudi, A. The costimulatory molecule CD226 signals through VAV1 to amplify TCR signals and promote IL-17 production by CD4(+) T cells Article de journal Dans: Sci Signal, vol. 11, no. 538, 2018, ISSN: 1937-9145 (Electronic)
1945-0877 (Linking). @article{RN30b,
title = {The costimulatory molecule CD226 signals through VAV1 to amplify TCR signals and promote IL-17 production by CD4(+) T cells},
author = {Gaud, G. and Roncagalli, R. and Chaoui, K. and Bernard, I. and Familiades, J. and Colacios, C. and Kassem, S. and Monsarrat, B. and Burlet-Schiltz, O. and de Peredo, A. G. and Malissen, B. and Saoudi, A.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29991650},
doi = {10.1126/scisignal.aar3083},
issn = {1937-9145 (Electronic)
1945-0877 (Linking)},
year = {2018},
date = {2018-01-01},
journal = {Sci Signal},
volume = {11},
number = {538},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2017
|
Yshii, L. M.; Hohlfeld, R.; Liblau, R. S. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives Article de journal Dans: Nat Rev Neurol, vol. 13, no. 12, p. 755-763, 2017, ISSN: 1759-4766 (Electronic)
1759-4758 (Linking). @article{RN23b,
title = {Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives},
author = {Yshii, L. M. and Hohlfeld, R. and Liblau, R. S.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29104289},
doi = {10.1038/nrneurol.2017.144},
issn = {1759-4766 (Electronic)
1759-4758 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Nat Rev Neurol},
volume = {13},
number = {12},
pages = {755-763},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yu, C.; Fitzpatrick, A.; Cong, D.; Yao, C.; Yoo, J.; Turnbull, A.; Schwarze, J.; Norval, M.; Howie, S. E. M.; Weller, R. B.; Astier, A. L. Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential Article de journal Dans: J Allergy Clin Immunol, 2017, ISSN: 1097-6825 (Electronic)
0091-6749 (Linking). @article{RN15b,
title = {Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential},
author = {Yu, C. and Fitzpatrick, A. and Cong, D. and Yao, C. and Yoo, J. and Turnbull, A. and Schwarze, J. and Norval, M. and Howie, S. E. M. and Weller, R. B. and Astier, A. L.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/28601680},
doi = {10.1016/j.jaci.2017.05.023},
issn = {1097-6825 (Electronic)
0091-6749 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {J Allergy Clin Immunol},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yshii, L. M.; Hohlfeld, R.; Liblau, R. S. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives Article de journal Dans: Nat Rev Neurol, vol. 13, no. 12, p. 755-763, 2017, ISSN: 1759-4766 (Electronic) 1759-4758 (Linking). @article{RN5b,
title = {Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives},
author = {Yshii, L. M. and Hohlfeld, R. and Liblau, R. S.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29104289},
doi = {10.1038/nrneurol.2017.144},
issn = {1759-4766 (Electronic) 1759-4758 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Nat Rev Neurol},
volume = {13},
number = {12},
pages = {755-763},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Ni Choileain, S.; Hay, J.; Thomas, J.; Williams, A.; Vermeren, M. M.; Benezech, C.; Gomez-Salazar, M.; Hugues, O. R.; Vermeren, S.; Howie, S. E. M.; Dransfield, I.; Astier, A. L. TCR-stimulated changes in cell surface CD46 expression generate type 1 regulatory T cells Article de journal Dans: Sci Signal, vol. 10, no. 502, 2017, ISSN: 1937-9145 (Electronic)
1945-0877 (Linking). @article{RN31b,
title = {TCR-stimulated changes in cell surface CD46 expression generate type 1 regulatory T cells},
author = {Ni Choileain, S. and Hay, J. and Thomas, J. and Williams, A. and Vermeren, M. M. and Benezech, C. and Gomez-Salazar, M. and Hugues, O. R. and Vermeren, S. and Howie, S. E. M. and Dransfield, I. and Astier, A. L.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29066539},
doi = {10.1126/scisignal.aah6163},
issn = {1937-9145 (Electronic)
1945-0877 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Sci Signal},
volume = {10},
number = {502},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Fissolo, N.; Pignolet, B.; Matute-Blanch, C.; Trivino, J. C.; Miro, B.; Mota, M.; Perez-Hoyos, S.; Sanchez, A.; Vermersch, P.; Ruet, A.; de Seze, J.; Labauge, P.; Vukusic, S.; Papeix, C.; Almoyna, L.; Tourbah, A.; Clavelou, P.; Moreau, T.; Pelletier, J.; Lebrun-Frenay, C.; Montalban, X.; Brassat, D.; Comabella, M.; Biomarkers,; Response to Natalizumab for Multiple Sclerosis Treatment, Best EScalation Treatment in Multiple Sclerosis; the Societe Francophone de la Sclerose En Plaques, Network Matrix metalloproteinase 9 is decreased in natalizumab-treated multiple sclerosis patients at risk for progressive multifocal leukoencephalopathy Article de journal Dans: Ann Neurol, vol. 82, no. 2, p. 186-195, 2017, ISSN: 1531-8249 (Electronic)
0364-5134 (Linking). @article{RN2b,
title = {Matrix metalloproteinase 9 is decreased in natalizumab-treated multiple sclerosis patients at risk for progressive multifocal leukoencephalopathy},
author = {Fissolo, N. and Pignolet, B. and Matute-Blanch, C. and Trivino, J. C. and Miro, B. and Mota, M. and Perez-Hoyos, S. and Sanchez, A. and Vermersch, P. and Ruet, A. and de Seze, J. and Labauge, P. and Vukusic, S. and Papeix, C. and Almoyna, L. and Tourbah, A. and Clavelou, P. and Moreau, T. and Pelletier, J. and Lebrun-Frenay, C. and Montalban, X. and Brassat, D. and Comabella, M. and Biomarkers and Response to Natalizumab for Multiple Sclerosis Treatment, Best EScalation Treatment in Multiple Sclerosis and the Societe Francophone de la Sclerose En Plaques, Network},
url = {http://www.ncbi.nlm.nih.gov/pubmed/28681388},
doi = {10.1002/ana.24987},
issn = {1531-8249 (Electronic)
0364-5134 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Ann Neurol},
volume = {82},
number = {2},
pages = {186-195},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yu, C.; Fitzpatrick, A.; Cong, D.; Yao, C.; Yoo, J.; Turnbull, A.; Schwarze, J.; Norval, M.; Howie, S. E. M.; Weller, R. B.; Astier, A. L. Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential Article de journal Dans: J Allergy Clin Immunol, 2017, ISSN: 1097-6825 (Electronic)
0091-6749 (Linking). @article{RN15b,
title = {Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential},
author = {Yu, C. and Fitzpatrick, A. and Cong, D. and Yao, C. and Yoo, J. and Turnbull, A. and Schwarze, J. and Norval, M. and Howie, S. E. M. and Weller, R. B. and Astier, A. L.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/28601680},
doi = {10.1016/j.jaci.2017.05.023},
issn = {1097-6825 (Electronic)
0091-6749 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {J Allergy Clin Immunol},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Gebauer, C.; Pignolet, B.; Yshii, L.; Maure, E.; Bauer, J.; Liblau, R. CD4+ and CD8+ T cells are both needed to induce paraneoplastic neurological disease in a mouse model Article de journal Dans: Oncoimmunology, vol. 6, no. 2, p. e1260212, 2017, ISSN: 2162-4011 (Print)
2162-4011 (Linking). @article{RN36b,
title = {CD4+ and CD8+ T cells are both needed to induce paraneoplastic neurological disease in a mouse model},
author = {Gebauer, C. and Pignolet, B. and Yshii, L. and Maure, E. and Bauer, J. and Liblau, R.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/28344867},
doi = {10.1080/2162402X.2016.1260212},
issn = {2162-4011 (Print)
2162-4011 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Oncoimmunology},
volume = {6},
number = {2},
pages = {e1260212},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Duguet, F.; Locard-Paulet, M.; Marcellin, M.; Chaoui, K.; Bernard, I.; Andreoletti, O.; Lesourne, R.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Saoudi, A. Proteomic Analysis of Regulatory T Cells Reveals the Importance of Themis1 in the Control of Their Suppressive Function Article de journal Dans: Mol Cell Proteomics, vol. 16, no. 8, p. 1416-1432, 2017, ISSN: 1535-9484 (Electronic)
1535-9476 (Linking). @article{RN35b,
title = {Proteomic Analysis of Regulatory T Cells Reveals the Importance of Themis1 in the Control of Their Suppressive Function},
author = {Duguet, F. and Locard-Paulet, M. and Marcellin, M. and Chaoui, K. and Bernard, I. and Andreoletti, O. and Lesourne, R. and Burlet-Schiltz, O. and Gonzalez de Peredo, A. and Saoudi, A.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/28373295},
doi = {10.1074/mcp.M116.062745},
issn = {1535-9484 (Electronic)
1535-9476 (Linking)},
year = {2017},
date = {2017-01-01},
journal = {Mol Cell Proteomics},
volume = {16},
number = {8},
pages = {1416-1432},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2016
|
Ramadan, A.; Lucca, L. E.; Carrie, N.; Desbois, S.; Axisa, P. P.; Hayder, M.; Bauer, J.; Liblau, R. S.; Mars, L. T. In situ expansion of T cells that recognize distinct self-antigens sustains autoimmunity in the CNS Article de journal Dans: Brain, vol. 139, no. Pt 5, p. 1433-46, 2016, ISSN: 1460-2156 (Electronic)
0006-8950 (Linking). @article{RN34b,
title = {In situ expansion of T cells that recognize distinct self-antigens sustains autoimmunity in the CNS},
author = {Ramadan, A. and Lucca, L. E. and Carrie, N. and Desbois, S. and Axisa, P. P. and Hayder, M. and Bauer, J. and Liblau, R. S. and Mars, L. T.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/27000832},
doi = {10.1093/brain/aww032},
issn = {1460-2156 (Electronic)
0006-8950 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Brain},
volume = {139},
number = {Pt 5},
pages = {1433-46},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Kassem, S.; Gaud, G.; Bernard, I.; Benamar, M.; Dejean, A. S.; Liblau, R.; Fournie, G. J.; Colacios, C.; Malissen, B.; Saoudi, A. A Natural Variant of the T Cell Receptor-Signaling Molecule Vav1 Reduces Both Effector T Cell Functions and Susceptibility to Neuroinflammation Article de journal Dans: PLoS Genet, vol. 12, no. 7, p. e1006185, 2016, ISSN: 1553-7404 (Electronic)
1553-7390 (Linking). @article{RN12b,
title = {A Natural Variant of the T Cell Receptor-Signaling Molecule Vav1 Reduces Both Effector T Cell Functions and Susceptibility to Neuroinflammation},
author = {Kassem, S. and Gaud, G. and Bernard, I. and Benamar, M. and Dejean, A. S. and Liblau, R. and Fournie, G. J. and Colacios, C. and Malissen, B. and Saoudi, A.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27438086},
doi = {10.1371/journal.pgen.1006185},
issn = {1553-7404 (Electronic)
1553-7390 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {PLoS Genet},
volume = {12},
number = {7},
pages = {e1006185},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Martin-Blondel, G.; Brassat, D.; Bauer, J.; Lassmann, H.; Liblau, R. S. CCR5 blockade for neuroinflammatory diseases--beyond control of HIV Article de journal Dans: Nat Rev Neurol, vol. 12, no. 2, p. 95-105, 2016, ISSN: 1759-4766 (Electronic)
1759-4758 (Linking). @article{RN4b,
title = {CCR5 blockade for neuroinflammatory diseases--beyond control of HIV},
author = {Martin-Blondel, G. and Brassat, D. and Bauer, J. and Lassmann, H. and Liblau, R. S.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26782333},
doi = {10.1038/nrneurol.2015.248},
issn = {1759-4766 (Electronic)
1759-4758 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Nat Rev Neurol},
volume = {12},
number = {2},
pages = {95-105},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pignolet, B.; Schwab, N.; Schneider-Hohendorf, T.; Bucciarelli, F.; Biotti, D.; Averseng-Peaureaux, D.; Outteryck, O.; Ongagna, J. C.; de Seze, J.; Brochet, B.; Ouallet, J. C.; Debouverie, M.; Pittion, S.; Defer, G.; Derache, N.; Hautecoeur, P.; Tourbah, A.; Labauge, P.; Castelnovo, G.; Clavelou, P.; Berger, E.; Pelletier, J.; Rico, A.; Zephir, H.; Laplaud, D.; Wiertlewski, S.; Camu, W.; Thouvenot, E.; Casez, O.; Moreau, T.; Fromont, A.; Vukusic, S.; Papeix, C.; Vermersch, P.; Comabella, M.; Lebrun-Frenay, C.; Wiendl, H.; Brassat, D.; Bionat, Sfsep; networks, Best-Ms CD62L test at 2 years of natalizumab predicts progressive multifocal leukoencephalopathy Article de journal Dans: Neurology, vol. 87, no. 23, p. 2491-2494, 2016, ISSN: 1526-632X (Electronic)
0028-3878 (Linking). @article{RN1,
title = {CD62L test at 2 years of natalizumab predicts progressive multifocal leukoencephalopathy},
author = {Pignolet, B. and Schwab, N. and Schneider-Hohendorf, T. and Bucciarelli, F. and Biotti, D. and Averseng-Peaureaux, D. and Outteryck, O. and Ongagna, J. C. and de Seze, J. and Brochet, B. and Ouallet, J. C. and Debouverie, M. and Pittion, S. and Defer, G. and Derache, N. and Hautecoeur, P. and Tourbah, A. and Labauge, P. and Castelnovo, G. and Clavelou, P. and Berger, E. and Pelletier, J. and Rico, A. and Zephir, H. and Laplaud, D. and Wiertlewski, S. and Camu, W. and Thouvenot, E. and Casez, O. and Moreau, T. and Fromont, A. and Vukusic, S. and Papeix, C. and Vermersch, P. and Comabella, M. and Lebrun-Frenay, C. and Wiendl, H. and Brassat, D. and Bionat, Sfsep and networks, Best-Ms},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27815407},
doi = {10.1212/WNL.0000000000003401},
issn = {1526-632X (Electronic)
0028-3878 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Neurology},
volume = {87},
number = {23},
pages = {2491-2494},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Schwab, N.; Schneider-Hohendorf, T.; Pignolet, B.; Spadaro, M.; Gorlich, D.; Meinl, I.; Windhagen, S.; Tackenberg, B.; Breuer, J.; Canto, E.; Kumpfel, T.; Hohlfeld, R.; Siffrin, V.; Luessi, F.; Posevitz-Fejfar, A.; Montalban, X.; Meuth, S. G.; Zipp, F.; Gold, R.; Du Pasquier, R. A.; Kleinschnitz, C.; Jacobi, A.; Comabella, M.; Bertolotto, A.; Brassat, D.; Wiendl, H. PML risk stratification using anti-JCV antibody index and L-selectin Article de journal Dans: Mult Scler, vol. 22, no. 8, p. 1048-60, 2016, ISSN: 1477-0970 (Electronic)
1352-4585 (Linking). @article{RN3b,
title = {PML risk stratification using anti-JCV antibody index and L-selectin},
author = {Schwab, N. and Schneider-Hohendorf, T. and Pignolet, B. and Spadaro, M. and Gorlich, D. and Meinl, I. and Windhagen, S. and Tackenberg, B. and Breuer, J. and Canto, E. and Kumpfel, T. and Hohlfeld, R. and Siffrin, V. and Luessi, F. and Posevitz-Fejfar, A. and Montalban, X. and Meuth, S. G. and Zipp, F. and Gold, R. and Du Pasquier, R. A. and Kleinschnitz, C. and Jacobi, A. and Comabella, M. and Bertolotto, A. and Brassat, D. and Wiendl, H.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26432858},
doi = {10.1177/1352458515607651},
issn = {1477-0970 (Electronic)
1352-4585 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Mult Scler},
volume = {22},
number = {8},
pages = {1048-60},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yshii, L. M.; Gebauer, C. M.; Pignolet, B.; Maure, E.; Queriault, C.; Pierau, M.; Saito, H.; Suzuki, N.; Brunner-Weinzierl, M.; Bauer, J.; Liblau, R. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model Article de journal Dans: Brain, 2016, ISSN: 1460-2156 (Electronic)
0006-8950 (Linking). @article{RN10b,
title = {CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model},
author = {Yshii, L. M. and Gebauer, C. M. and Pignolet, B. and Maure, E. and Queriault, C. and Pierau, M. and Saito, H. and Suzuki, N. and Brunner-Weinzierl, M. and Bauer, J. and Liblau, R.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27604307},
doi = {10.1093/brain/aww225},
issn = {1460-2156 (Electronic)
0006-8950 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Brain},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bernard-Valnet, R.; Yshii, L.; Queriault, C.; Nguyen, X. H.; Arthaud, S.; Rodrigues, M.; Canivet, A.; Morel, A. L.; Matthys, A.; Bauer, J.; Pignolet, B.; Dauvilliers, Y.; Peyron, C.; Liblau, R. S. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice Article de journal Dans: Proc Natl Acad Sci U S A, vol. 113, no. 39, p. 10956-61, 2016, ISSN: 1091-6490 (Electronic) 0027-8424 (Linking). @article{RN9b,
title = {CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice},
author = {Bernard-Valnet, R. and Yshii, L. and Queriault, C. and Nguyen, X. H. and Arthaud, S. and Rodrigues, M. and Canivet, A. and Morel, A. L. and Matthys, A. and Bauer, J. and Pignolet, B. and Dauvilliers, Y. and Peyron, C. and Liblau, R. S.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27621438},
doi = {10.1073/pnas.1603325113},
issn = {1091-6490 (Electronic) 0027-8424 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Proc Natl Acad Sci U S A},
volume = {113},
number = {39},
pages = {10956-61},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Aloulou, M.; Carr, E. J.; Gador, M.; Bignon, A.; Liblau, R. S.; Fazilleau, N.; Linterman, M. A. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells Article de journal Dans: Nat Commun, vol. 7, p. 10579, 2016, ISSN: 2041-1723 (Electronic)
2041-1723 (Linking). @article{RN37b,
title = {Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells},
author = {Aloulou, M. and Carr, E. J. and Gador, M. and Bignon, A. and Liblau, R. S. and Fazilleau, N. and Linterman, M. A.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/26818004},
doi = {10.1038/ncomms10579},
issn = {2041-1723 (Electronic)
2041-1723 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Nat Commun},
volume = {7},
pages = {10579},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Yshii, L. M.; Gebauer, C. M.; Pignolet, B.; Maure, E.; Queriault, C.; Pierau, M.; Saito, H.; Suzuki, N.; Brunner-Weinzierl, M.; Bauer, J.; Liblau, R. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model Article de journal Dans: Brain, 2016, ISSN: 1460-2156 (Electronic)
0006-8950 (Linking). @article{RN10b,
title = {CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model},
author = {Yshii, L. M. and Gebauer, C. M. and Pignolet, B. and Maure, E. and Queriault, C. and Pierau, M. and Saito, H. and Suzuki, N. and Brunner-Weinzierl, M. and Bauer, J. and Liblau, R.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27604307},
doi = {10.1093/brain/aww225},
issn = {1460-2156 (Electronic)
0006-8950 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Brain},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Stienne, C.; Michieletto, M. F.; Benamar, M.; Carrie, N.; Bernard, I.; Nguyen, X. H.; Lippi, Y.; Duguet, F.; Liblau, R. S.; Hedrick, S. M.; Saoudi, A.; Dejean, A. S. Foxo3 Transcription Factor Drives Pathogenic T Helper 1 Differentiation by Inducing the Expression of Eomes Article de journal Dans: Immunity, vol. 45, no. 4, p. 774-787, 2016, ISSN: 1097-4180 (Electronic)
1074-7613 (Linking). @article{RN17b,
title = {Foxo3 Transcription Factor Drives Pathogenic T Helper 1 Differentiation by Inducing the Expression of Eomes},
author = {Stienne, C. and Michieletto, M. F. and Benamar, M. and Carrie, N. and Bernard, I. and Nguyen, X. H. and Lippi, Y. and Duguet, F. and Liblau, R. S. and Hedrick, S. M. and Saoudi, A. and Dejean, A. S.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27742544},
doi = {10.1016/j.immuni.2016.09.010},
issn = {1097-4180 (Electronic)
1074-7613 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Immunity},
volume = {45},
number = {4},
pages = {774-787},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Mansuy, J. M.; Suberbielle, E.; Chapuy-Regaud, S.; Mengelle, C.; Bujan, L.; Marchou, B.; Delobel, P.; Gonzalez-Dunia, D.; Malnou, C. E.; Izopet, J.; Martin-Blondel, G. Zika virus in semen and spermatozoa Article de journal Dans: Lancet Infect Dis, vol. 16, no. 10, p. 1106-1107, 2016, ISSN: 1474-4457 (Electronic)
1473-3099 (Linking). @article{RN28b,
title = {Zika virus in semen and spermatozoa},
author = {Mansuy, J. M. and Suberbielle, E. and Chapuy-Regaud, S. and Mengelle, C. and Bujan, L. and Marchou, B. and Delobel, P. and Gonzalez-Dunia, D. and Malnou, C. E. and Izopet, J. and Martin-Blondel, G.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/27676340},
doi = {10.1016/S1473-3099(16)30336-X},
issn = {1474-4457 (Electronic)
1473-3099 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Lancet Infect Dis},
volume = {16},
number = {10},
pages = {1106-1107},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Mansuy, J. M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Article de journal Dans: Lancet Infect Dis, vol. 16, no. 4, p. 405, 2016, ISSN: 1474-4457 (Electronic)
1473-3099 (Linking). @article{RN29b,
title = {Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen?},
author = {Mansuy, J. M. and Dutertre, M. and Mengelle, C. and Fourcade, C. and Marchou, B. and Delobel, P. and Izopet, J. and Martin-Blondel, G.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/26949027},
doi = {10.1016/S1473-3099(16)00138-9},
issn = {1474-4457 (Electronic)
1473-3099 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Lancet Infect Dis},
volume = {16},
number = {4},
pages = {405},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Hartmann, F. J.; Bernard-Valnet, R.; Queriault, C.; Mrdjen, D.; Weber, L. M.; Galli, E.; Krieg, C.; Robinson, M. D.; Nguyen, X. H.; Dauvilliers, Y.; Liblau, R. S.; Becher, B. High-dimensional single-cell analysis reveals the immune signature of narcolepsy Article de journal Dans: J Exp Med, vol. 213, no. 12, p. 2621-2633, 2016, ISSN: 1540-9538 (Electronic)
0022-1007 (Linking). @article{RN11b,
title = {High-dimensional single-cell analysis reveals the immune signature of narcolepsy},
author = {Hartmann, F. J. and Bernard-Valnet, R. and Queriault, C. and Mrdjen, D. and Weber, L. M. and Galli, E. and Krieg, C. and Robinson, M. D. and Nguyen, X. H. and Dauvilliers, Y. and Liblau, R. S. and Becher, B.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27821550},
doi = {10.1084/jem.20160897},
issn = {1540-9538 (Electronic)
0022-1007 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {J Exp Med},
volume = {213},
number = {12},
pages = {2621-2633},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Kieback, E.; Hilgenberg, E.; Stervbo, U.; Lampropoulou, V.; Shen, P.; Bunse, M.; Jaimes, Y.; Boudinot, P.; Radbruch, A.; Klemm, U.; Kuhl, A. A.; Liblau, R.; Hoevelmeyer, N.; Anderton, S. M.; Uckert, W.; Fillatreau, S. Thymus-Derived Regulatory T Cells Are Positively Selected on Natural Self-Antigen through Cognate Interactions of High Functional Avidity Article de journal Dans: Immunity, vol. 44, no. 5, p. 1114-26, 2016, ISSN: 1097-4180 (Electronic)
1074-7613 (Linking). @article{RN38,
title = {Thymus-Derived Regulatory T Cells Are Positively Selected on Natural Self-Antigen through Cognate Interactions of High Functional Avidity},
author = {Kieback, E. and Hilgenberg, E. and Stervbo, U. and Lampropoulou, V. and Shen, P. and Bunse, M. and Jaimes, Y. and Boudinot, P. and Radbruch, A. and Klemm, U. and Kuhl, A. A. and Liblau, R. and Hoevelmeyer, N. and Anderton, S. M. and Uckert, W. and Fillatreau, S.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/27192577},
doi = {10.1016/j.immuni.2016.04.018},
issn = {1097-4180 (Electronic)
1074-7613 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {Immunity},
volume = {44},
number = {5},
pages = {1114-26},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Hartmann, F. J.; Bernard-Valnet, R.; Queriault, C.; Mrdjen, D.; Weber, L. M.; Galli, E.; Krieg, C.; Robinson, M. D.; Nguyen, X. H.; Dauvilliers, Y.; Liblau, R. S.; Becher, B. High-dimensional single-cell analysis reveals the immune signature of narcolepsy Article de journal Dans: J Exp Med, vol. 213, no. 12, p. 2621-2633, 2016, ISSN: 1540-9538 (Electronic)
0022-1007 (Linking). @article{RN39b,
title = {High-dimensional single-cell analysis reveals the immune signature of narcolepsy},
author = {Hartmann, F. J. and Bernard-Valnet, R. and Queriault, C. and Mrdjen, D. and Weber, L. M. and Galli, E. and Krieg, C. and Robinson, M. D. and Nguyen, X. H. and Dauvilliers, Y. and Liblau, R. S. and Becher, B.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/27821550},
doi = {10.1084/jem.20160897},
issn = {1540-9538 (Electronic)
0022-1007 (Linking)},
year = {2016},
date = {2016-01-01},
journal = {J Exp Med},
volume = {213},
number = {12},
pages = {2621-2633},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2015
|
Martin-Blondel, G.; Bauer, J.; Uro-Coste, E.; Biotti, D.; Averseng-Peaureaux, D.; Fabre, N.; Dumas, H.; Bonneville, F.; Lassmann, H.; Marchou, B.; Liblau, R. S.; Brassat, D. Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome Article de journal Dans: Acta Neuropathol, vol. 129, no. 3, p. 463-5, 2015, ISSN: 1432-0533 (Electronic)
0001-6322 (Linking). @article{RN25b,
title = {Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome},
author = {Martin-Blondel, G. and Bauer, J. and Uro-Coste, E. and Biotti, D. and Averseng-Peaureaux, D. and Fabre, N. and Dumas, H. and Bonneville, F. and Lassmann, H. and Marchou, B. and Liblau, R. S. and Brassat, D.},
url = {https://www.ncbi.nlm.nih.gov/pubmed/25589222},
doi = {10.1007/s00401-015-1383-6},
issn = {1432-0533 (Electronic)
0001-6322 (Linking)},
year = {2015},
date = {2015-01-01},
journal = {Acta Neuropathol},
volume = {129},
number = {3},
pages = {463-5},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Hafler, D. A.; Astier, A. L. Editorial: T cell regulation by the environment Article de journal Dans: Front Immunol, vol. 6, p. 229, 2015, ISSN: 1664-3224 (Print)
1664-3224 (Linking). @article{RN16b,
title = {Editorial: T cell regulation by the environment},
author = {Hafler, D. A. and Astier, A. L.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26029214},
doi = {10.3389/fimmu.2015.00229},
issn = {1664-3224 (Print)
1664-3224 (Linking)},
year = {2015},
date = {2015-01-01},
journal = {Front Immunol},
volume = {6},
pages = {229},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Martin-Blondel, G.; Bauer, J.; Uro-Coste, E.; Biotti, D.; Averseng-Peaureaux, D.; Fabre, N.; Dumas, H.; Bonneville, F.; Lassmann, H.; Marchou, B.; Liblau, R. S.; Brassat, D. Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome Article de journal Dans: Acta Neuropathol, vol. 129, no. 3, p. 463-5, 2015, ISSN: 1432-0533 (Electronic)
0001-6322 (Linking). @article{RN5b,
title = {Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome},
author = {Martin-Blondel, G. and Bauer, J. and Uro-Coste, E. and Biotti, D. and Averseng-Peaureaux, D. and Fabre, N. and Dumas, H. and Bonneville, F. and Lassmann, H. and Marchou, B. and Liblau, R. S. and Brassat, D.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/25589222},
doi = {10.1007/s00401-015-1383-6},
issn = {1432-0533 (Electronic)
0001-6322 (Linking)},
year = {2015},
date = {2015-01-01},
journal = {Acta Neuropathol},
volume = {129},
number = {3},
pages = {463-5},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pedros, C.; Gaud, G.; Bernard, I.; Kassem, S.; Chabod, M.; Lagrange, D.; Andreoletti, O.; Dejean, A. S.; Lesourne, R.; Fournie, G. J.; Saoudi, A. An Epistatic Interaction between Themis1 and Vav1 Modulates Regulatory T Cell Function and Inflammatory Bowel Disease Development Article de journal Dans: J Immunol, vol. 195, no. 4, p. 1608-16, 2015, ISSN: 1550-6606 (Electronic)
0022-1767 (Linking). @article{RN13b,
title = {An Epistatic Interaction between Themis1 and Vav1 Modulates Regulatory T Cell Function and Inflammatory Bowel Disease Development},
author = {Pedros, C. and Gaud, G. and Bernard, I. and Kassem, S. and Chabod, M. and Lagrange, D. and Andreoletti, O. and Dejean, A. S. and Lesourne, R. and Fournie, G. J. and Saoudi, A.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26163585},
doi = {10.4049/jimmunol.1402562},
issn = {1550-6606 (Electronic)
0022-1767 (Linking)},
year = {2015},
date = {2015-01-01},
journal = {J Immunol},
volume = {195},
number = {4},
pages = {1608-16},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2014
|
Outteryck, O.; Ongagna, J. C.; Brochet, B.; Rumbach, L.; Lebrun-Frenay, C.; Debouverie, M.; Zephir, H.; Ouallet, J. C.; Berger, E.; Cohen, M.; Pittion, S.; Laplaud, D.; Wiertlewski, S.; Cabre, P.; Pelletier, J.; Rico, A.; Defer, G.; Derache, N.; Camu, W.; Thouvenot, E.; Moreau, T.; Fromont, A.; Tourbah, A.; Labauge, P.; Castelnovo, G.; Clavelou, P.; Casez, O.; Hautecoeur, P.; Papeix, C.; Lubetzki, C.; Fontaine, B.; Couturier, N.; Bohossian, N.; Clanet, M.; Vermersch, P.; de Seze, J.; Brassat, D.; Network, Bionat; Cfsep, A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: clinical, radiological and biological features and adverse events. The BIONAT cohort Article de journal Dans: Eur J Neurol, vol. 21, no. 1, p. 40-8, 2014, ISSN: 1468-1331 (Electronic)
1351-5101 (Linking). @article{RN6b,
title = {A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: clinical, radiological and biological features and adverse events. The BIONAT cohort},
author = {Outteryck, O. and Ongagna, J. C. and Brochet, B. and Rumbach, L. and Lebrun-Frenay, C. and Debouverie, M. and Zephir, H. and Ouallet, J. C. and Berger, E. and Cohen, M. and Pittion, S. and Laplaud, D. and Wiertlewski, S. and Cabre, P. and Pelletier, J. and Rico, A. and Defer, G. and Derache, N. and Camu, W. and Thouvenot, E. and Moreau, T. and Fromont, A. and Tourbah, A. and Labauge, P. and Castelnovo, G. and Clavelou, P. and Casez, O. and Hautecoeur, P. and Papeix, C. and Lubetzki, C. and Fontaine, B. and Couturier, N. and Bohossian, N. and Clanet, M. and Vermersch, P. and de Seze, J. and Brassat, D. and Network, Bionat and Cfsep},
url = {http://www.ncbi.nlm.nih.gov/pubmed/23895407},
doi = {10.1111/ene.12204},
issn = {1468-1331 (Electronic)
1351-5101 (Linking)},
year = {2014},
date = {2014-01-01},
journal = {Eur J Neurol},
volume = {21},
number = {1},
pages = {40-8},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2011
|
International Multiple Sclerosis Genetics, Consortium; Wellcome Trust Case Control, Consortium; Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C. C.; Patsopoulos, N. A.; Moutsianas, L.; Dilthey, A.; Su, Z.; Freeman, C.; Hunt, S. E.; Edkins, S.; Gray, E.; Booth, D. R.; Potter, S. C.; Goris, A.; Band, G.; Oturai, A. B.; Strange, A.; Saarela, J.; Bellenguez, C.; Fontaine, B.; Gillman, M.; Hemmer, B.; Gwilliam, R.; Zipp, F.; Jayakumar, A.; Martin, R.; Leslie, S.; Hawkins, S.; Giannoulatou, E.; D'Alfonso, S.; Blackburn, H.; Martinelli Boneschi, F.; Liddle, J.; Harbo, H. F.; Perez, M. L.; Spurkland, A.; Waller, M. J.; Mycko, M. P.; Ricketts, M.; Comabella, M.; Hammond, N.; Kockum, I.; McCann, O. T.; Ban, M.; Whittaker, P.; Kemppinen, A.; Weston, P.; Hawkins, C.; Widaa, S.; Zajicek, J.; Dronov, S.; Robertson, N.; Bumpstead, S. J.; Barcellos, L. F.; Ravindrarajah, R.; Abraham, R.; Alfredsson, L.; Ardlie, K.; Aubin, C.; Baker, A.; Baker, K.; Baranzini, S. E.; Bergamaschi, L.; Bergamaschi, R.; Bernstein, A.; Berthele, A.; Boggild, M.; Bradfield, J. P.; Brassat, D.; Broadley, S. A.; Buck, D.; Butzkueven, H.; Capra, R.; Carroll, W. M.; Cavalla, P.; Celius, E. G.; Cepok, S.; Chiavacci, R.; Clerget-Darpoux, F.; Clysters, K.; Comi, G.; Cossburn, M.; Cournu-Rebeix, I.; Cox, M. B.; Cozen, W.; Cree, B. A.; Cross, A. H.; Cusi, D.; Daly, M. J.; Davis, E.; de Bakker, P. I.; Debouverie, M.; D'Hooghe M, B.; Dixon, K.; Dobosi, R.; Dubois, B.; Ellinghaus, D.; others, Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis Article de journal Dans: Nature, vol. 476, no. 7359, p. 214-9, 2011, ISSN: 1476-4687 (Electronic)
0028-0836 (Linking). @article{RN7b,
title = {Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis},
author = {International Multiple Sclerosis Genetics, Consortium and Wellcome Trust Case Control, Consortium and Sawcer, S. and Hellenthal, G. and Pirinen, M. and Spencer, C. C. and Patsopoulos, N. A. and Moutsianas, L. and Dilthey, A. and Su, Z. and Freeman, C. and Hunt, S. E. and Edkins, S. and Gray, E. and Booth, D. R. and Potter, S. C. and Goris, A. and Band, G. and Oturai, A. B. and Strange, A. and Saarela, J. and Bellenguez, C. and Fontaine, B. and Gillman, M. and Hemmer, B. and Gwilliam, R. and Zipp, F. and Jayakumar, A. and Martin, R. and Leslie, S. and Hawkins, S. and Giannoulatou, E. and D'Alfonso, S. and Blackburn, H. and Martinelli Boneschi, F. and Liddle, J. and Harbo, H. F. and Perez, M. L. and Spurkland, A. and Waller, M. J. and Mycko, M. P. and Ricketts, M. and Comabella, M. and Hammond, N. and Kockum, I. and McCann, O. T. and Ban, M. and Whittaker, P. and Kemppinen, A. and Weston, P. and Hawkins, C. and Widaa, S. and Zajicek, J. and Dronov, S. and Robertson, N. and Bumpstead, S. J. and Barcellos, L. F. and Ravindrarajah, R. and Abraham, R. and Alfredsson, L. and Ardlie, K. and Aubin, C. and Baker, A. and Baker, K. and Baranzini, S. E. and Bergamaschi, L. and Bergamaschi, R. and Bernstein, A. and Berthele, A. and Boggild, M. and Bradfield, J. P. and Brassat, D. and Broadley, S. A. and Buck, D. and Butzkueven, H. and Capra, R. and Carroll, W. M. and Cavalla, P. and Celius, E. G. and Cepok, S. and Chiavacci, R. and Clerget-Darpoux, F. and Clysters, K. and Comi, G. and Cossburn, M. and Cournu-Rebeix, I. and Cox, M. B. and Cozen, W. and Cree, B. A. and Cross, A. H. and Cusi, D. and Daly, M. J. and Davis, E. and de Bakker, P. I. and Debouverie, M. and D'Hooghe M, B. and Dixon, K. and Dobosi, R. and Dubois, B. and Ellinghaus, D. and others},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21833088},
doi = {10.1038/nature10251},
issn = {1476-4687 (Electronic)
0028-0836 (Linking)},
year = {2011},
date = {2011-01-01},
journal = {Nature},
volume = {476},
number = {7359},
pages = {214-9},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Dejean, A. S.; Hedrick, S. M.; Kerdiles, Y. M. Highly specialized role of Forkhead box O transcription factors in the immune system Article de journal Dans: Antioxid Redox Signal, vol. 14, no. 4, p. 663-74, 2011, ISSN: 1557-7716 (Electronic)
1523-0864 (Linking). @article{RN18b,
title = {Highly specialized role of Forkhead box O transcription factors in the immune system},
author = {Dejean, A. S. and Hedrick, S. M. and Kerdiles, Y. M.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/20673126},
doi = {10.1089/ars.2010.3414},
issn = {1557-7716 (Electronic)
1523-0864 (Linking)},
year = {2011},
date = {2011-01-01},
journal = {Antioxid Redox Signal},
volume = {14},
number = {4},
pages = {663-74},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Couturier, N.; Bucciarelli, F.; Nurtdinov, R. N.; Debouverie, M.; Lebrun-Frenay, C.; Defer, G.; Moreau, T.; Confavreux, C.; Vukusic, S.; Cournu-Rebeix, I.; Goertsches, R. H.; Zettl, U. K.; Comabella, M.; Montalban, X.; Rieckmann, P.; Weber, F.; Muller-Myhsok, B.; Edan, G.; Fontaine, B.; Mars, L. T.; Saoudi, A.; Oksenberg, J. R.; Clanet, M.; Liblau, R. S.; Brassat, D. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility Article de journal Dans: Brain, vol. 134, no. Pt 3, p. 693-703, 2011, ISSN: 1460-2156 (Electronic)
0006-8950 (Linking). @article{RN8b,
title = {Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility},
author = {Couturier, N. and Bucciarelli, F. and Nurtdinov, R. N. and Debouverie, M. and Lebrun-Frenay, C. and Defer, G. and Moreau, T. and Confavreux, C. and Vukusic, S. and Cournu-Rebeix, I. and Goertsches, R. H. and Zettl, U. K. and Comabella, M. and Montalban, X. and Rieckmann, P. and Weber, F. and Muller-Myhsok, B. and Edan, G. and Fontaine, B. and Mars, L. T. and Saoudi, A. and Oksenberg, J. R. and Clanet, M. and Liblau, R. S. and Brassat, D.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21354972},
doi = {10.1093/brain/awr010},
issn = {1460-2156 (Electronic)
0006-8950 (Linking)},
year = {2011},
date = {2011-01-01},
journal = {Brain},
volume = {134},
number = {Pt 3},
pages = {693-703},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Colacios, C.; Casemayou, A.; Dejean, A. S.; Gaits-Iacovoni, F.; Pedros, C.; Bernard, I.; Lagrange, D.; Deckert, M.; Lamouroux, L.; Jagodic, M.; Olsson, T.; Liblau, R. S.; Fournie, G. J.; Saoudi, A. The p.Arg63Trp polymorphism controls Vav1 functions and Foxp3 regulatory T cell development Article de journal Dans: J Exp Med, vol. 208, no. 11, p. 2183-91, 2011, ISSN: 1540-9538 (Electronic)
0022-1007 (Linking). @article{RN14b,
title = {The p.Arg63Trp polymorphism controls Vav1 functions and Foxp3 regulatory T cell development},
author = {Colacios, C. and Casemayou, A. and Dejean, A. S. and Gaits-Iacovoni, F. and Pedros, C. and Bernard, I. and Lagrange, D. and Deckert, M. and Lamouroux, L. and Jagodic, M. and Olsson, T. and Liblau, R. S. and Fournie, G. J. and Saoudi, A.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21948080},
doi = {10.1084/jem.20102191},
issn = {1540-9538 (Electronic)
0022-1007 (Linking)},
year = {2011},
date = {2011-01-01},
journal = {J Exp Med},
volume = {208},
number = {11},
pages = {2183-91},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2009
|
Dejean, A. S.; Beisner, D. R.; Ch'en, I. L.; Kerdiles, Y. M.; Babour, A.; Arden, K. C.; Castrillon, D. H.; DePinho, R. A.; Hedrick, S. M. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells Article de journal Dans: Nat Immunol, vol. 10, no. 5, p. 504-13, 2009, ISSN: 1529-2916 (Electronic)
1529-2908 (Linking). @article{RN19,
title = {Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells},
author = {Dejean, A. S. and Beisner, D. R. and Ch'en, I. L. and Kerdiles, Y. M. and Babour, A. and Arden, K. C. and Castrillon, D. H. and DePinho, R. A. and Hedrick, S. M.},
url = {http://www.ncbi.nlm.nih.gov/pubmed/19363483},
doi = {10.1038/ni.1729},
issn = {1529-2916 (Electronic)
1529-2908 (Linking)},
year = {2009},
date = {2009-01-01},
journal = {Nat Immunol},
volume = {10},
number = {5},
pages = {504-13},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
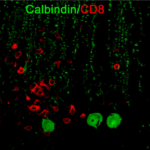 Nos projets de recherche reposent sur l’utilisation de modèles expérimentaux uniques et d’échantillons biologiques de patients. Nous étudions la transmigration et les fonctions effectrices des lymphocytes T, en particulier des lymphocytes T CD8, en utilisant plusieurs modèles d’auto-immunité du SNC que nous avons récemment développés dans le laboratoire. Plus précisément :
Nos projets de recherche reposent sur l’utilisation de modèles expérimentaux uniques et d’échantillons biologiques de patients. Nous étudions la transmigration et les fonctions effectrices des lymphocytes T, en particulier des lymphocytes T CD8, en utilisant plusieurs modèles d’auto-immunité du SNC que nous avons récemment développés dans le laboratoire. Plus précisément :
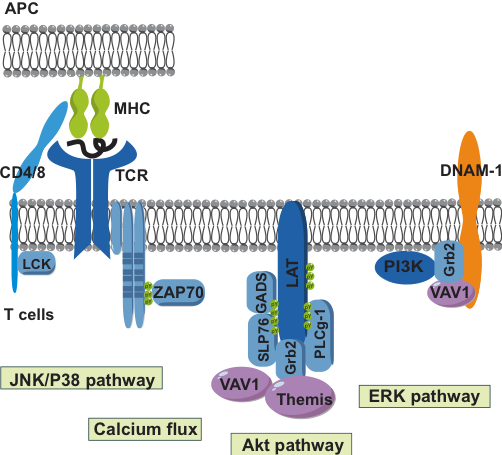 À cet égard, nos études récentes ont révélé l’implication de Vav1, Themis1, CD46 et CD226 dans les fonctions des cellules T et la susceptibilité aux troubles immunitaires. Cependant, les mécanismes cellulaires et moléculaires par lesquels ces molécules de signalisation coopèrent pour réguler la fonction des cellules T restent inconnus.
À cet égard, nos études récentes ont révélé l’implication de Vav1, Themis1, CD46 et CD226 dans les fonctions des cellules T et la susceptibilité aux troubles immunitaires. Cependant, les mécanismes cellulaires et moléculaires par lesquels ces molécules de signalisation coopèrent pour réguler la fonction des cellules T restent inconnus.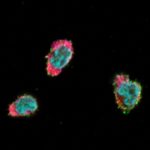
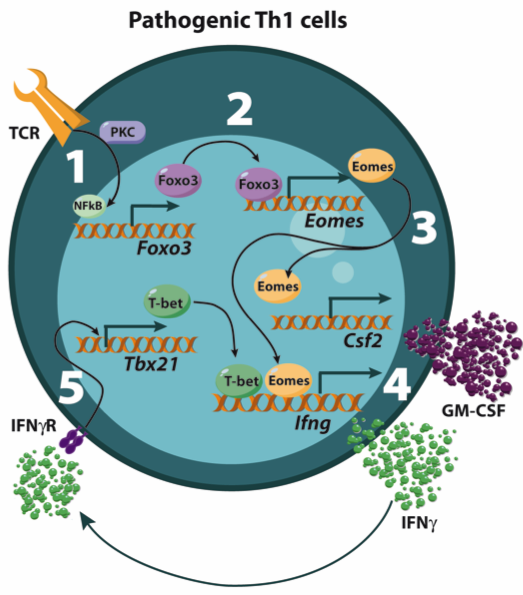 Nous avons récemment démontré que le facteur de transcription Foxo3 joue un rôle clé dans la physiopathologie de l’auto-immunité du SNC puisque la déficience en Foxo3 est associée à une diminution significative de la sévérité de l’EAE. Nous avons également mis en évidence que cette diminution résultait de l’incapacité des lymphocytes T CD4+ déficients en Foxo3 à se différencier en cellules T helper-1 (Th1) pathogènes, caractérisés par la production d’Interféron-g (IFN-g) et du facteur de stimulation des colonies de granulocytes-macrophages (GM-CSF). Au niveau moléculaire, nous avons également mis en évidence quela voie Foxo3-Eomes est essentielle pour la mise en place du programme génétique nécessaire pour la différenciation des cellules Th1 pathogènes et le développement de la neuroinflammation.
Nous avons récemment démontré que le facteur de transcription Foxo3 joue un rôle clé dans la physiopathologie de l’auto-immunité du SNC puisque la déficience en Foxo3 est associée à une diminution significative de la sévérité de l’EAE. Nous avons également mis en évidence que cette diminution résultait de l’incapacité des lymphocytes T CD4+ déficients en Foxo3 à se différencier en cellules T helper-1 (Th1) pathogènes, caractérisés par la production d’Interféron-g (IFN-g) et du facteur de stimulation des colonies de granulocytes-macrophages (GM-CSF). Au niveau moléculaire, nous avons également mis en évidence quela voie Foxo3-Eomes est essentielle pour la mise en place du programme génétique nécessaire pour la différenciation des cellules Th1 pathogènes et le développement de la neuroinflammation.









