2025
|
Collercandy, Nived; Vellas, Camille; Nayrac, Manon; Requena, Mary; Richarme, Thomas; Iscache, Anne-Laure; Latour, Justine; Barange, Karl; Alric, Laurent; Martin-Blondel, Guillaume; Serino, Matteo; Izopet, Jacques; Delobel, Pierre Cytotoxic CX3CR1+ Vδ1 T cells clonally expand in an interplay of CMV, microbiota, and HIV-1 persistence in people on antiretroviral therapy Article de journal Dans: PLoS Pathog, vol. 21, no. 9, p. e1013489, 2025, ISSN: 1553-7374. @article{pmid40920867,
title = {Cytotoxic CX3CR1+ Vδ1 T cells clonally expand in an interplay of CMV, microbiota, and HIV-1 persistence in people on antiretroviral therapy},
author = {Nived Collercandy and Camille Vellas and Manon Nayrac and Mary Requena and Thomas Richarme and Anne-Laure Iscache and Justine Latour and Karl Barange and Laurent Alric and Guillaume Martin-Blondel and Matteo Serino and Jacques Izopet and Pierre Delobel},
doi = {10.1371/journal.ppat.1013489},
issn = {1553-7374},
year = {2025},
date = {2025-09-01},
urldate = {2025-09-01},
journal = {PLoS Pathog},
volume = {21},
number = {9},
pages = {e1013489},
abstract = {Vδ1 γδ T cells are key players in innate and adaptive immunity, particularly at mucosal interfaces such as the gut. An increase in circulating Vδ1 cells has long been observed in people with HIV-1, but remains poorly understood. We performed a comprehensive characterization of Vδ1 T cells in blood and duodenal intra-epithelial lymphocytes, obtained from endoscopic mucosal biopsies of 15 people with HIV-1 on antiretroviral therapy and 15 HIV-seronegative controls, in a substudy of the ANRS EP61 GALT study (NCT02906137). We deciphered the phenotype, functional profile, single-cell transcriptome and repertoire of Vδ1 cells and unraveled their relationships with the possible triggers involved, in particular CMV and microbiota. We also assessed whether Vδ1 T cells may play a role in controlling the HIV-1 reservoir. Vδ1 T cells were mainly terminally differentiated effectors that clonally expanded in the blood with some trafficking with the gut of people with HIV-1. Most expressed CX3CR1 and displayed a highly cytotoxic profile, but low cytokine production, supported by a transcriptomic shift towards enhanced effector lymphocytes. This expansion was associated with CMV status and markers of occult replication, but also with changes in the duodenal and blood-translocated microbiota. Cytotoxic, but not IFN-γ-producing, Vδ1 T cells were negatively associated with cell-associated HIV-1 RNA in both the blood and duodenal compartments. The increase in Vδ1 T cells observed in people with HIV-1 has multiple triggers, particularly CMV and microbiota, and may in turn contribute to the control of the HIV-1 reservoir.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Vδ1 γδ T cells are key players in innate and adaptive immunity, particularly at mucosal interfaces such as the gut. An increase in circulating Vδ1 cells has long been observed in people with HIV-1, but remains poorly understood. We performed a comprehensive characterization of Vδ1 T cells in blood and duodenal intra-epithelial lymphocytes, obtained from endoscopic mucosal biopsies of 15 people with HIV-1 on antiretroviral therapy and 15 HIV-seronegative controls, in a substudy of the ANRS EP61 GALT study (NCT02906137). We deciphered the phenotype, functional profile, single-cell transcriptome and repertoire of Vδ1 cells and unraveled their relationships with the possible triggers involved, in particular CMV and microbiota. We also assessed whether Vδ1 T cells may play a role in controlling the HIV-1 reservoir. Vδ1 T cells were mainly terminally differentiated effectors that clonally expanded in the blood with some trafficking with the gut of people with HIV-1. Most expressed CX3CR1 and displayed a highly cytotoxic profile, but low cytokine production, supported by a transcriptomic shift towards enhanced effector lymphocytes. This expansion was associated with CMV status and markers of occult replication, but also with changes in the duodenal and blood-translocated microbiota. Cytotoxic, but not IFN-γ-producing, Vδ1 T cells were negatively associated with cell-associated HIV-1 RNA in both the blood and duodenal compartments. The increase in Vδ1 T cells observed in people with HIV-1 has multiple triggers, particularly CMV and microbiota, and may in turn contribute to the control of the HIV-1 reservoir. |
Belloy, Marcy; Schmitt, Benjamin A M; Marty, Florent H; Paut, Charlotte; Bassot, Emilie; Aïda, Amel; Alis, Marine; Zahm, Margot; Chaubet, Adeline; Garnier, Hugo; Flores-Aguilar, Thelma; Roitg, Elisa; Gutierrez-Loli, Renzo; Allart, Sophie; Ecalard, Romain; Boursereau, Raphaël; Ligat, Gaëtan; Gonzalez-Dunia, Daniel; Blanchard, Nicolas; Suberbielle, Elsa Toxoplasma gondii infection and chronic IL-1 elevation drive hippocampal DNA double-strand break signaling, leading to cognitive deficits Article de journal Dans: Nat Neurosci, 2025, ISSN: 1546-1726. @article{pmid40841478,
title = {Toxoplasma gondii infection and chronic IL-1 elevation drive hippocampal DNA double-strand break signaling, leading to cognitive deficits},
author = {Marcy Belloy and Benjamin A M Schmitt and Florent H Marty and Charlotte Paut and Emilie Bassot and Amel Aïda and Marine Alis and Margot Zahm and Adeline Chaubet and Hugo Garnier and Thelma Flores-Aguilar and Elisa Roitg and Renzo Gutierrez-Loli and Sophie Allart and Romain Ecalard and Raphaël Boursereau and Gaëtan Ligat and Daniel Gonzalez-Dunia and Nicolas Blanchard and Elsa Suberbielle},
doi = {10.1038/s41593-025-02041-x},
issn = {1546-1726},
year = {2025},
date = {2025-08-01},
urldate = {2025-08-01},
journal = {Nat Neurosci},
abstract = {Chronic inflammation, resulting from infections, is characterized by increased levels of cytokines including interleukin-1 (IL-1), but little is known about how IL-1 contributes to cognitive impairment, potentially via epigenetic processes. Here we demonstrate that mice chronically infected with the parasite Toxoplasma gondii exhibit impaired spatial memory, which is dependent on neuronal IL-1 signaling and mimicked by chronic exposure to IL-1β. Both T. gondii infection and chronic IL-1β drive H2A.X-dependent DNA double-strand break signaling in hippocampal neurons and invalidating neuronal H2A.X-dependent signaling blocks memory impairments caused by either exposure. Our results highlight the instrumental role of cytokine-induced double-strand-break-dependent signaling in spatial memory defects, which may be relevant to multiple brain diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Chronic inflammation, resulting from infections, is characterized by increased levels of cytokines including interleukin-1 (IL-1), but little is known about how IL-1 contributes to cognitive impairment, potentially via epigenetic processes. Here we demonstrate that mice chronically infected with the parasite Toxoplasma gondii exhibit impaired spatial memory, which is dependent on neuronal IL-1 signaling and mimicked by chronic exposure to IL-1β. Both T. gondii infection and chronic IL-1β drive H2A.X-dependent DNA double-strand break signaling in hippocampal neurons and invalidating neuronal H2A.X-dependent signaling blocks memory impairments caused by either exposure. Our results highlight the instrumental role of cytokine-induced double-strand-break-dependent signaling in spatial memory defects, which may be relevant to multiple brain diseases. |
2024
|
Maire, Kilian; Chamy, Léa; Ghazali, Samira; Carratala-Lasserre, Manon; Zahm, Margot; Bouisset, Clément; Métais, Arnaud; Combes-Soia, Lucie; Fuente-Vizuete, Lidia; Trad, Hussein; Chaubet, Adeline; Savignac, Magali; de Peredo, Anne Gonzalez; Subramaniam, Arun; Joffre, Olivier; Lutz, Pierre G.; Lamsoul, Isabelle Fine-tuning levels of filamins a and b as a specific mechanism sustaining Th2 lymphocyte functions Article de journal Dans: Nature Communications, vol. 15, no. 1, p. 10574, 2024, ISSN: 2041-1723. @article{maire_fine-tuning_2024,
title = {Fine-tuning levels of filamins a and b as a specific mechanism sustaining Th2 lymphocyte functions},
author = {Kilian Maire and Léa Chamy and Samira Ghazali and Manon Carratala-Lasserre and Margot Zahm and Clément Bouisset and Arnaud Métais and Lucie Combes-Soia and Lidia Fuente-Vizuete and Hussein Trad and Adeline Chaubet and Magali Savignac and Anne Gonzalez de Peredo and Arun Subramaniam and Olivier Joffre and Pierre G. Lutz and Isabelle Lamsoul},
doi = {10.1038/s41467-024-53768-3},
issn = {2041-1723},
year = {2024},
date = {2024-12-01},
urldate = {2024-12-01},
journal = {Nature Communications},
volume = {15},
number = {1},
pages = {10574},
abstract = {Augmenting the portfolio of therapeutics for type 2-driven diseases is crucial to address unmet clinical needs and to design personalized treatment schemes. An attractive therapy for such diseases would consist in targeting the recruitment of T helper 2 (Th2) lymphocytes to inflammatory sites. Herein, we show the degradation of filamins (FLN) a and b by the ASB2α E3 ubiquitin ligase as a mechanism sustaining Th2 lymphocyte functions. Low levels of FLNa and FLNb confer an elongated shape to Th2 lymphocytes associated with efficient αVβ3 integrin-dependent cell migration. Genes encoding the αVβ3 integrin and ASB2α belong to the core of Th2-specific genes. Using genetically modified mice, we find that increasing the levels of FLNa and FLNb in Th2 lymphocytes reduces airway inflammation through diminished Th2 lymphocyte recruitment in inflamed lungs. Collectively, our results highlight ASB2α and its substrates FLNa and FLNb to alter Th2 lymphocyte-mediated responses.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Augmenting the portfolio of therapeutics for type 2-driven diseases is crucial to address unmet clinical needs and to design personalized treatment schemes. An attractive therapy for such diseases would consist in targeting the recruitment of T helper 2 (Th2) lymphocytes to inflammatory sites. Herein, we show the degradation of filamins (FLN) a and b by the ASB2α E3 ubiquitin ligase as a mechanism sustaining Th2 lymphocyte functions. Low levels of FLNa and FLNb confer an elongated shape to Th2 lymphocytes associated with efficient αVβ3 integrin-dependent cell migration. Genes encoding the αVβ3 integrin and ASB2α belong to the core of Th2-specific genes. Using genetically modified mice, we find that increasing the levels of FLNa and FLNb in Th2 lymphocytes reduces airway inflammation through diminished Th2 lymphocyte recruitment in inflamed lungs. Collectively, our results highlight ASB2α and its substrates FLNa and FLNb to alter Th2 lymphocyte-mediated responses. |
Dias, Chloé; Ballout, Nissrine; Morla, Guillaume; Alileche, Katia; Santiago, Christophe; Guerrera, Ida Chiara; Chaubet, Adeline; Ausseil, Jerome; Trudel, Stephanie Extracellular vesicles from microglial cells activated by abnormal heparan sulfate oligosaccharides from Sanfilippo patients impair neuronal dendritic arborization Article de journal Dans: Molecular Medicine, vol. 30, no. 1, p. 197, 2024, ISSN: 1528-3658. @article{nokey,
title = {Extracellular vesicles from microglial cells activated by abnormal heparan sulfate oligosaccharides from Sanfilippo patients impair neuronal dendritic arborization},
author = {Chloé Dias and Nissrine Ballout and Guillaume Morla and Katia Alileche and Christophe Santiago and Ida Chiara Guerrera and Adeline Chaubet and Jerome Ausseil and Stephanie Trudel},
doi = {10.1186/s10020-024-00953-1},
issn = {1528-3658},
year = {2024},
date = {2024-11-04},
journal = {Molecular Medicine},
volume = {30},
number = {1},
pages = {197},
abstract = {Background
In mucopolysaccharidosis type III (MPS III, also known as Sanfilippo syndrome), a pediatric neurodegenerative disorder, accumulation of abnormal glycosaminoglycans (GAGs) induces severe neuroinflammation by triggering the microglial pro-inflammatory cytokines production via a TLR4-dependent pathway. But the extent of the microglia contribution to the MPS III neuropathology remains unclear. Extracellular vesicles (EVs) mediate intercellular communication and are known to participate in the pathogenesis of adult neurodegenerative diseases. However, characterization of the molecular profiles of EVs released by MPS III microglia and their effects on neuronal functions have not been described.
Methods
Here, we isolated EVs secreted by the microglial cells after treatment with GAGs purified from urines of Sanfilippo patients (sfGAGs-EVs) or from age-matched healthy subjects (nGAGs-EVs) to explore the EVs’ proteins and small RNA profiles using LC–MS/MS and RNA sequencing. We next performed a functional assay by immunofluorescence following nGAGs- or sfGAGs-EVs uptake by WT primary cortical neurons and analyzed their extensions metrics after staining of βIII-tubulin and MAP2 by confocal microscopy.
Results
Functional enrichment analysis for both proteomics and RNA sequencing data from sfGAGs-EVs revealed a specific content involved in neuroinflammation and neurodevelopment pathways. Treatment of cortical neurons with sfGAGs-EVs induced a disease-associated phenotype demonstrated by a lower total neurite surface area, an impaired somatodendritic compartment, and a higher number of immature dendritic spines.
Conclusions
This study shows, for the first time, that GAGs from patients with Sanfilippo syndrome can induce microglial secretion of EVs that deliver a specific molecular message to recipient naive neurons, while promoting the neuroinflammation, and depriving neurons of neurodevelopmental factors. This work provides a framework for further studies of biomarkers to evaluate efficiency of emerging therapies.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Background
In mucopolysaccharidosis type III (MPS III, also known as Sanfilippo syndrome), a pediatric neurodegenerative disorder, accumulation of abnormal glycosaminoglycans (GAGs) induces severe neuroinflammation by triggering the microglial pro-inflammatory cytokines production via a TLR4-dependent pathway. But the extent of the microglia contribution to the MPS III neuropathology remains unclear. Extracellular vesicles (EVs) mediate intercellular communication and are known to participate in the pathogenesis of adult neurodegenerative diseases. However, characterization of the molecular profiles of EVs released by MPS III microglia and their effects on neuronal functions have not been described.
Methods
Here, we isolated EVs secreted by the microglial cells after treatment with GAGs purified from urines of Sanfilippo patients (sfGAGs-EVs) or from age-matched healthy subjects (nGAGs-EVs) to explore the EVs’ proteins and small RNA profiles using LC–MS/MS and RNA sequencing. We next performed a functional assay by immunofluorescence following nGAGs- or sfGAGs-EVs uptake by WT primary cortical neurons and analyzed their extensions metrics after staining of βIII-tubulin and MAP2 by confocal microscopy.
Results
Functional enrichment analysis for both proteomics and RNA sequencing data from sfGAGs-EVs revealed a specific content involved in neuroinflammation and neurodevelopment pathways. Treatment of cortical neurons with sfGAGs-EVs induced a disease-associated phenotype demonstrated by a lower total neurite surface area, an impaired somatodendritic compartment, and a higher number of immature dendritic spines.
Conclusions
This study shows, for the first time, that GAGs from patients with Sanfilippo syndrome can induce microglial secretion of EVs that deliver a specific molecular message to recipient naive neurons, while promoting the neuroinflammation, and depriving neurons of neurodevelopmental factors. This work provides a framework for further studies of biomarkers to evaluate efficiency of emerging therapies. |
Morandi, Elena; Adoue, Véronique; Bernard, Isabelle; Friebel, Ekaterina; Nunez, Nicolas; Aubert, Yann; Masson, Frederick; Dejean, Anne S; Becher, Burkhard; Astier, Anne; Martinet, Ludovic; Saoudi, Abdelhadi Impact of the Multiple Sclerosis-Associated Genetic Variant CD226 Gly307Ser on Human CD8 T-Cell Functions Article de journal Dans: Neurol Neuroimmunol Neuroinflammation, vol. 11, no. 6, 2024. @article{nokey,
title = {Impact of the Multiple Sclerosis-Associated Genetic Variant CD226 Gly307Ser on Human CD8 T-Cell Functions},
author = {Elena Morandi and Véronique Adoue and Isabelle Bernard and Ekaterina Friebel and Nicolas Nunez and Yann Aubert and Frederick Masson and Anne S Dejean and Burkhard Becher and Anne Astier and Ludovic Martinet and Abdelhadi Saoudi},
doi = {10.1212/NXI.0000000000200306},
year = {2024},
date = {2024-09-04},
urldate = {2024-09-04},
journal = {Neurol Neuroimmunol Neuroinflammation},
volume = {11},
number = {6},
abstract = {Background and Objectives
The rs763361 nonsynonymous variant in the CD226 gene, which results in a glycine-to-serine substitution at position 307 of the CD226 protein, has been implicated as a risk factor of various immune-mediated diseases, including multiple sclerosis (MS). Compelling evidence suggests that this allele may play a significant role in predisposing individuals to MS by decreasing the immune-regulatory capacity of Treg cells and increasing the proinflammatory potential of effector CD4 T cells. However, the impact of this CD226 gene variant on CD8 T-cell functions, a population that also plays a key role in MS, remains to be determined.
Methods
To study whether the CD226 risk variant affects human CD8 T-cell functions, we used CD8 T cells isolated from peripheral blood mononuclear cell of 16 age-matched healthy donors homozygous for either the protective or the risk allele of CD226. We characterized these CD8 T cells on T-cell receptor (TCR) stimulation using high-parametric flow cytometry and bulk RNAseq and through characterization of canonical signaling pathways and cytokine production.
Results
On TCR engagement, the phenotype of ex vivo CD8 T cells bearing the protective (CD226-307Gly) or the risk (CD226-307Ser) allele of CD226 was largely overlapping. However, the transcriptomic signature of CD8 T cells from the donors carrying the risk allele presented an enrichment in TCR, JAK/STAT, and IFNγ signaling. We next found that the CD226-307Ser risk allele leads to a selective increase in the phosphorylation of the mitogen-activated protein kinases extracellular signal–regulated kinases 1 and 2 (ERK1/2) associated with enhanced phosphorylation of STAT4 and increased production of IFNγ.
Discussion
Our data suggest that the CD226-307Ser risk variant imposes immune dysregulation by increasing the pathways related to IFNγ signaling in CD8 T cells, thereby contributing to the risk of developing chronic inflammation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Background and Objectives
The rs763361 nonsynonymous variant in the CD226 gene, which results in a glycine-to-serine substitution at position 307 of the CD226 protein, has been implicated as a risk factor of various immune-mediated diseases, including multiple sclerosis (MS). Compelling evidence suggests that this allele may play a significant role in predisposing individuals to MS by decreasing the immune-regulatory capacity of Treg cells and increasing the proinflammatory potential of effector CD4 T cells. However, the impact of this CD226 gene variant on CD8 T-cell functions, a population that also plays a key role in MS, remains to be determined.
Methods
To study whether the CD226 risk variant affects human CD8 T-cell functions, we used CD8 T cells isolated from peripheral blood mononuclear cell of 16 age-matched healthy donors homozygous for either the protective or the risk allele of CD226. We characterized these CD8 T cells on T-cell receptor (TCR) stimulation using high-parametric flow cytometry and bulk RNAseq and through characterization of canonical signaling pathways and cytokine production.
Results
On TCR engagement, the phenotype of ex vivo CD8 T cells bearing the protective (CD226-307Gly) or the risk (CD226-307Ser) allele of CD226 was largely overlapping. However, the transcriptomic signature of CD8 T cells from the donors carrying the risk allele presented an enrichment in TCR, JAK/STAT, and IFNγ signaling. We next found that the CD226-307Ser risk allele leads to a selective increase in the phosphorylation of the mitogen-activated protein kinases extracellular signal–regulated kinases 1 and 2 (ERK1/2) associated with enhanced phosphorylation of STAT4 and increased production of IFNγ.
Discussion
Our data suggest that the CD226-307Ser risk variant imposes immune dysregulation by increasing the pathways related to IFNγ signaling in CD8 T cells, thereby contributing to the risk of developing chronic inflammation. |
Joulia, Emeline; Michieletto, Michaël F.; Agesta, Arantxa; Peillex, Cindy; Girault, Virginie; Dorze, Anne-Louise Le; Peroceschi, Romain; Bucciarelli, Florence; Szelechowski, Marion; Chaubet, Adeline; Hakim, Nawad; Marrocco, Rémi; Lhuillier, Emeline; Lebeurrier, Manuel; Argüello, Rafael J.; Saoudi, Abdelhadi; Costa, Hicham El; Adoue, Veronique; Walzer, Thierry; Sarry, Jean-Emmanuel; Dejean, Anne S. Eomes-dependent mitochondrial regulation promotes survival of pathogenic CD4+ T cells during inflammation Article de journal Dans: Journal of Experimental Medicine, vol. 221, no. 2, 2024, ISSN: 0022-1007. @article{nokey,
title = {Eomes-dependent mitochondrial regulation promotes survival of pathogenic CD4+ T cells during inflammation},
author = {Emeline Joulia and Michaël F. Michieletto and Arantxa Agesta and Cindy Peillex and Virginie Girault and Anne-Louise Le Dorze and Romain Peroceschi and Florence Bucciarelli and Marion Szelechowski and Adeline Chaubet and Nawad Hakim and Rémi Marrocco and Emeline Lhuillier and Manuel Lebeurrier and Rafael J. Argüello and Abdelhadi Saoudi and Hicham El Costa and Veronique Adoue and Thierry Walzer and Jean-Emmanuel Sarry and Anne S. Dejean},
doi = {10.1084/jem.20230449},
issn = {0022-1007},
year = {2024},
date = {2024-01-08},
journal = {Journal of Experimental Medicine},
volume = {221},
number = {2},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
2023
|
Osma-Garcia, Ines C.; Mouysset, Mailys; Capitan-Sobrino, Dunja; Aubert, Yann; Turner, Martin; Diaz-Muñoz, Manuel D. The RNA binding proteins TIA1 and TIAL1 promote Mcl1 mRNA translation to protect germinal center responses from apoptosis Article de journal Dans: Cellular & Molecular Immunology, 2023, ISSN: 2042-0226. @article{Osma-Garcia2023b,
title = {The RNA binding proteins TIA1 and TIAL1 promote Mcl1 mRNA translation to protect germinal center responses from apoptosis},
author = {Osma-Garcia, Ines C.
and Mouysset, Mailys
and Capitan-Sobrino, Dunja
and Aubert, Yann
and Turner, Martin
and Diaz-Mu{ñ}oz, Manuel D.},
url = {https://doi.org/10.1038/s41423-023-01063-4},
doi = {10.1038/s41423-023-01063-4},
issn = {2042-0226},
year = {2023},
date = {2023-07-20},
urldate = {2023-07-20},
journal = {Cellular & Molecular Immunology},
abstract = {Germinal centers (GCs) are essential for the establishment of long-lasting antibody responses. GC B cells rely on post-transcriptional RNA mechanisms to translate activation-associated transcriptional programs into functional changes in the cell proteome. However, the critical proteins driving these key mechanisms are still unknown. Here, we show that the RNA binding proteins TIA1 and TIAL1 are required for the generation of long-lasting GC responses. TIA1- and TIAL1-deficient GC B cells fail to undergo antigen-mediated positive selection, expansion and differentiation into B-cell clones producing high-affinity antibodies. Mechanistically, TIA1 and TIAL1 control the transcriptional identity of dark- and light-zone GC B cells and enable timely expression of the prosurvival molecule MCL1. Thus, we demonstrate here that TIA1 and TIAL1 are key players in the post-transcriptional program that selects high-affinity antigen-specific GC B cells.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Germinal centers (GCs) are essential for the establishment of long-lasting antibody responses. GC B cells rely on post-transcriptional RNA mechanisms to translate activation-associated transcriptional programs into functional changes in the cell proteome. However, the critical proteins driving these key mechanisms are still unknown. Here, we show that the RNA binding proteins TIA1 and TIAL1 are required for the generation of long-lasting GC responses. TIA1- and TIAL1-deficient GC B cells fail to undergo antigen-mediated positive selection, expansion and differentiation into B-cell clones producing high-affinity antibodies. Mechanistically, TIA1 and TIAL1 control the transcriptional identity of dark- and light-zone GC B cells and enable timely expression of the prosurvival molecule MCL1. Thus, we demonstrate here that TIA1 and TIAL1 are key players in the post-transcriptional program that selects high-affinity antigen-specific GC B cells. |
2018
|
Dupain, Célia; Harttrampf, Anne C.; Boursin, Yannick; Lebeurrier, Manuel; Rondof, Windy; Robert-Siegwald, Guillaume; Khoueiry, Pierre; Geoerger, Birgit; Massaad-Massade, Liliane Discovery of new fusion transcripts in a cohort of pediatric solid cancers at relapse and relevance for personalized medicine Article de journal Dans: Molecular Therapy, 2018. @article{CéliaDupain2018,
title = {Discovery of new fusion transcripts in a cohort of pediatric solid cancers at relapse and relevance for personalized medicine},
author = {Célia Dupain and Anne C. Harttrampf and Yannick Boursin and Manuel Lebeurrier and Windy Rondof and Guillaume Robert-Siegwald and Pierre Khoueiry and Birgit Geoerger and Liliane Massaad-Massade},
editor = {Cell Press},
url = {https://www.sciencedirect.com/science/article/pii/S152500161830529X},
doi = {https://doi.org/10.1016/j.ymthe.2018.10.022},
year = {2018},
date = {2018-11-02},
urldate = {2018-11-02},
journal = {Molecular Therapy},
abstract = {We hypothetized that pediatric cancers would more likely harbor fusion transcripts. To dissect the complexity of the fusions landscape in recurrent solid pediatric cancers, we conducted a study on 48 patients with different relapsing/resistant malignancies. By analysing RNA-sequencing data with a new in-house pipeline for fusions detection named ChimComp, followed by verification by real time PCR, we identified and classified the most confident fusion transcripts (FT) according to their potential biological function and druggability. The majority of FT were predicted to affect key cancer pathways and described to be involved in oncogenesis. Contrary to previous descriptions, we found no significant correlation between the number of fusions and mutations, emphasizing the particularity to study pre-treated pediatric patients. A considerable proportion of FT containing tumor suppressor genes was detected, reflecting their importance in pediatric cancers. FT containing non-receptor tyrosine kinases occurred at low incidence and predominantly in brain tumors. Remarkably, more than 30% of patients presented a potentially druggable high-confidence fusion. In conclusion, we detected new oncogenic FT in relapsing pediatric cancer patients by establishing a robust pipeline that can be applied to other malignancies, to detect and prioritize experimental validation studies leading to the development of new therapeutic options.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
We hypothetized that pediatric cancers would more likely harbor fusion transcripts. To dissect the complexity of the fusions landscape in recurrent solid pediatric cancers, we conducted a study on 48 patients with different relapsing/resistant malignancies. By analysing RNA-sequencing data with a new in-house pipeline for fusions detection named ChimComp, followed by verification by real time PCR, we identified and classified the most confident fusion transcripts (FT) according to their potential biological function and druggability. The majority of FT were predicted to affect key cancer pathways and described to be involved in oncogenesis. Contrary to previous descriptions, we found no significant correlation between the number of fusions and mutations, emphasizing the particularity to study pre-treated pediatric patients. A considerable proportion of FT containing tumor suppressor genes was detected, reflecting their importance in pediatric cancers. FT containing non-receptor tyrosine kinases occurred at low incidence and predominantly in brain tumors. Remarkably, more than 30% of patients presented a potentially druggable high-confidence fusion. In conclusion, we detected new oncogenic FT in relapsing pediatric cancer patients by establishing a robust pipeline that can be applied to other malignancies, to detect and prioritize experimental validation studies leading to the development of new therapeutic options. |
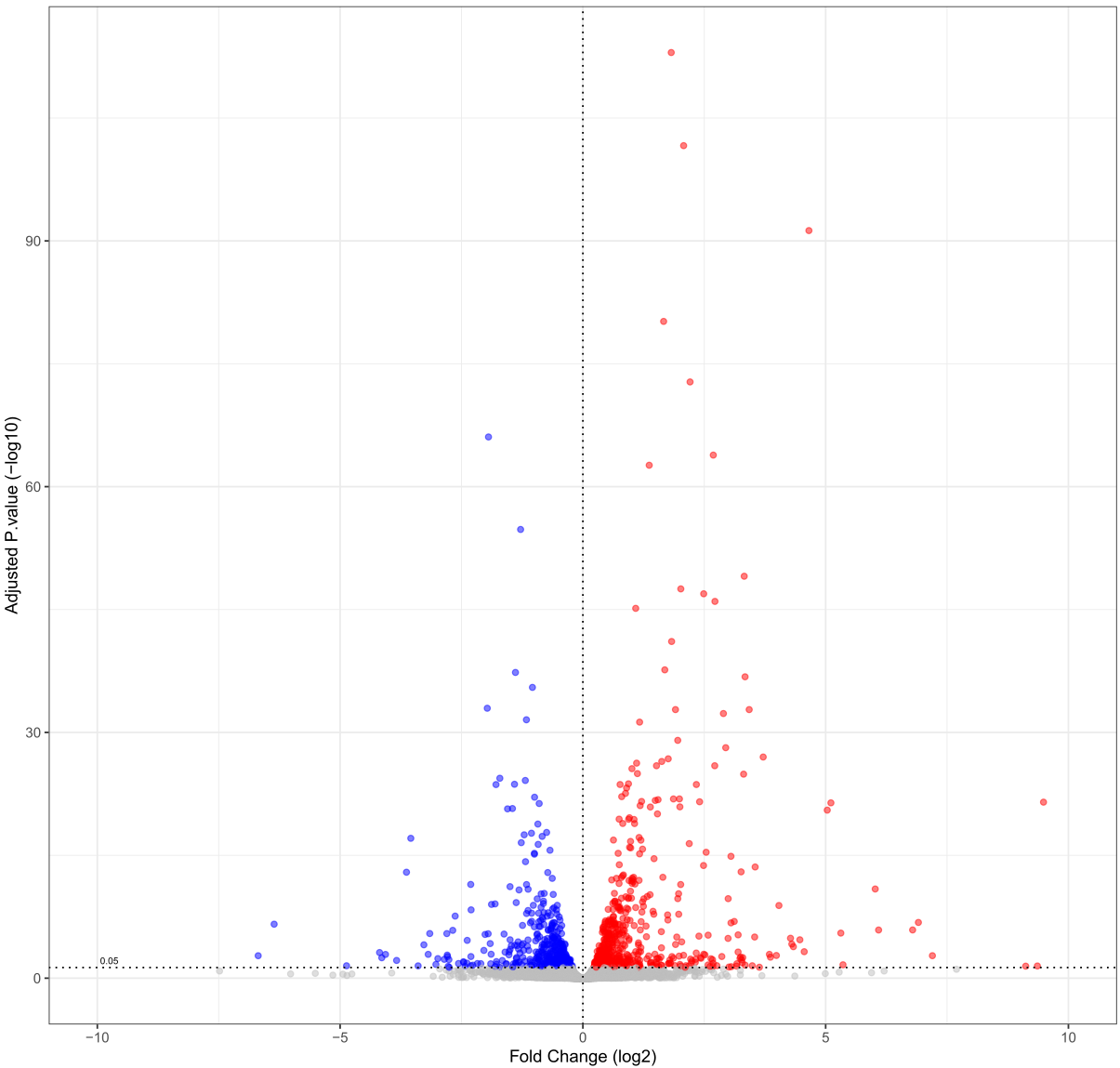 Le plateau réalise des analyses différentielles, analyses d’enrichissement, recherche de motifs ou tout autres analyses statistiques nécessaires dans le cadre de votre projet.
Le plateau réalise des analyses différentielles, analyses d’enrichissement, recherche de motifs ou tout autres analyses statistiques nécessaires dans le cadre de votre projet.